��Ŀ����
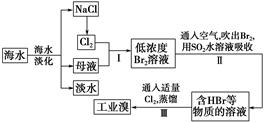
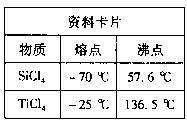
��ҵ�ϳ��ú�����SiO2��Al2O3�ĸ�����FeO Cr2O3��ұ��������Ҫ�������£�
Cr2O3��ұ��������Ҫ�������£�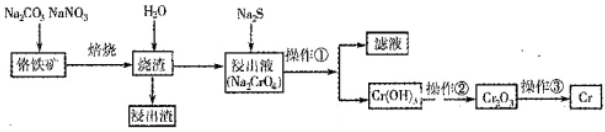
��1��������л�ѧ����ʽ���ں�����д���ʵĻ�ѧʽ��ϵ������
2FeO��Cr2O3��4Na2CO3��7NaNO3 4Na2CrO4��Fe2O3��4CO2��______________��
4Na2CrO4��Fe2O3��4CO2��______________��
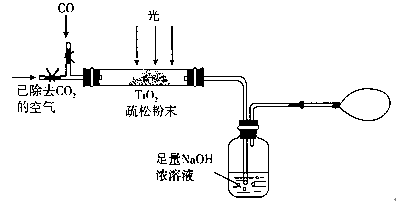
��2�������ٰ���������ϴ�ӣ���ʵ�����н���ϴ�ӳ����IJ���__________________________________�������ڿ�ѡ�õ�װ�ã����ּг�װ����ȥ����________������ţ�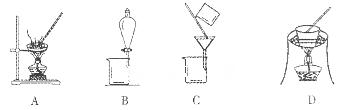
��3��д���ܹ���ɲ����۵���ط�Ӧ�Ļ�ѧ����ʽ��_____________________��
��4����ѧ��������COD���ɶ���ˮ�����л�����Ⱦ�ij̶ȡ���ǿ�Ტ���ȵ������£���K2Cr2O7��ǿ����������ˮ�������ⶨ���ĵ�K2Cr2O7������Ȼ������൱��O2�ĺ�����Ϊ��ѧ����������mg/L�ƣ�����ѧ��ȤС��ⶨijˮ���Ļ�ѧ��������COD���������£�
I.ȡamLˮ��������ƿ������10��00mL 0.2500 mol/L��K2Cr2O7��Һ��
II��������
III����ָʾ������c mol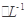 �����������[ ��NH4��2Fe��SO4��
�����������[ ��NH4��2Fe��SO4��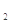 ]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3����
]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3����
��I����ȡK2Cr2O7��Һ��������_____________��
�ڼ����ˮ���Ļ�ѧ������ʱ���õ����й�ϵ��Ҫ��ȥ1molCr2O72�� ������___molFe2����1molCr2O72���൱��_______ molO2��
��1��7NaNO2��2�֣�
��2����©���м�����ˮ����û��������Һ�����£��ظ�����2��3�Σ�2�֣� D��2�֣�
��3��2C2O3 4Cr��3O2����2Al��C2O3
4Cr��3O2����2Al��C2O3 2Cr��Al2O3��2�֣������𰸺���Ҳ���֣�
2Cr��Al2O3��2�֣������𰸺���Ҳ���֣�
��4������ʽ�ζ��ܻ���Һ�ܣ�1�֣� ��6 1.5��ÿ��1�֣���2�֣�
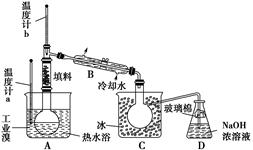
�����������: ��1�����ݷ�Ӧǰ��Ԫ�صĻ��ϼ۱仯��֪����Ӧǰ��Ԫ�صĻ��ϼ۴ӣ�2�����ߵ���3�ۣ�ʧȥ1�����ӡ�CrԪ�صĻ��ϼ۴ӣ�3�����ߵ���6�ۣ�ʧȥ3�����ӡ���O��Na��C�Ļ��ϼ۾�û�б仯�����Ը��ݵ��ӵĵ�ʧ�غ��֪����Ԫ�صĻ��ϼ�һ�����͡�������Ԫ�ع�ʧȥ2�����ӣ�CrԪ�ع�ʧȥ12�����ӡ����7����ԭ�ӵõ�14�����ӣ���Ԫ�صĻ��ϼ۴ӣ�5�۽��͵���3�ۣ����Ի�ԭ������NaNO2��
��2�����˺�ij���ϴ�����ڹ���������ɵģ�������ʵ�����н���ϴ�ӳ����IJ�������©���м�����ˮ����û��������Һ�����£��ظ�����2��3�Σ���������Cr��OH��3�ֽ�����Cr2O3��������ȷֽ��������н��С�A��������B�Ƿ�Һ��C�ǹ��ˣ���˴�ѡD��
��3��CrԪ���Ǹ��۵����������ͨ�����ȷ�Ӧ���ⷨұ������Ӧ�ķ���ʽ�ֱ�Ϊ2C2O3 4Cr��3O2����2Al��C2O3
4Cr��3O2����2Al��C2O3 2Cr��Al2O3��
2Cr��Al2O3��
��4����K2Cr2O7��Һ�������Ҿ���ǿ�����ԣ������Ҫ����ʽ�ζ��ܻ���Һ����ȡ��
��K2Cr2O7��������ԭ��Ӧ��CrԪ�صĻ��ϼ۴ӣ�6�۽��͵���3�ۣ��õ�3�����ӡ���������������Ԫ�صĻ��ϼ۴ӣ�2�����ߵ���3�ۣ�ʧȥ3�����ӡ���˸��ݵ��ӵ�ʧ�غ��֪��ȥ1molCr2O72�� ������6molFe2��������1mol�������������õ�4mol���ӣ�����molCr2O72���൱��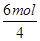 ��1.5molO2��
��1.5molO2��
���㣺�Թ�������Ϊ���忼��������ԭ��Ӧ����ʽ����ƽ�����㣻����ϴ�ӡ�Һ�����ȡ������Cr��ұ��

 ��У����ϵ�д�
��У����ϵ�д�����������(��Ҫ��Fe2O3��SiO2��Al2O3��MgO������)�Ʊ��������Ĺ����������£�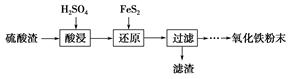
(1)�������������Ҫ�ʵ�������Ŀ���ǣ���������Ľ����ʣ���________��
(2)����ԭ���ǽ�Fe3��ת��ΪFe2����ͬʱFeS2������ΪSO42�����÷�Ӧ�����ӷ���ʽΪ________________________________________________________________________��
(3)Ϊ�ⶨ��������������Һ��Fe3�������Կ��Ƽ���FeS2������ʵ�鲽��Ϊ��ȷ��ȡһ���������������Һ����ƿ�У�����ϡ���ᡢ�Թ���SnCl2���ټ�HgCl2��ȥ������SnCl2���Զ�����������Ϊָʾ������K2Cr2O7����Һ�ζ����йط�Ӧ�Ļ�ѧ����ʽ���£�
2Fe3����Sn2����6Cl��=2Fe2����SnCl62��
Sn2����4Cl����2HgCl2=SnCl62����Hg2Cl2��
6Fe2����Cr2O72����14H��=6Fe3����2Cr3����7H2O
����SnCl2����������ⶨ��Fe3����________(�ƫ�ߡ�����ƫ�͡����䡱����ͬ)��
��������HgCl2����ⶨ��Fe3����________��
(4)�ٿ�ѡ��________(���Լ�)������Һ�к���Fe3��������Fe3����ԭ����________________________________________________________________________(�����ӷ���ʽ��ʾ)��
����֪����������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe(OH)3 | Al(OH)3 | Fe(OH)2 | Mg(OH)2 | Mn(OH)2 |
| ��ʼ���� | 2.7 | 3.8 | 7.5 | 9.4 | 8.3 |
| ��ȫ���� | 3.2 | 5.2 | 9.7 | 12.4 | 9.8 |
ʵ���ѡ�õ��Լ��У�ϡ���ᡢBa(NO3)2��Һ������KMnO4��Һ��NaOH��Һ��Ҫ���Ʊ������в������ж����塣
������ɡ����ˡ������Һģ���Ʊ���������ʵ�鲽�裺
a��������________________________________________________________��
b��������__________________________________________________________��
c�����룬ϴ�ӣ�
d����ɣ���ĥ��
���������û�������������������ų��ķ�������Ҫ��ѧ�ɷ�ΪSiO2Լ45%��Fe2O3Լ40%��Al2O3Լ10%��MgOԼ5%��Ŀǰ�ҹ��Ѿ��ڼ�����ȡ��ͻ�ơ������������з�������ֳɷֲ��������á������̺�����������£�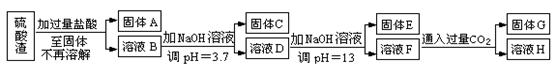
�����ϵ�֪��
| �������� | �ܶȻ�(Ksp) | pHֵ | |
| ��ʼ���� | ��ȫ���� | ||
| Mg(OH)2 | 5.6��10��12 | 9.3 | 10.8 |
| Fe(OH)3 | 2.8��10��16 | 2.7 | 3.7 |
| Al(OH)3 | 1.3��10��33 | 3.7 | 4.7 |
��ش��������⣺
��1��д������A�Ļ�ѧʽΪ ��
��2����Ҫ�ⶨ��Һ��pH�Ƿ�ﵽ3.7������ʵ����Ʒ�п�ѡ�õ��� ��
A��ʯ����Һ B���㷺pH��ֽ C������pH��ֽ D��pH��
��3������������ӷ�Ӧ����ʽ
����ҺD���ɹ���E �� ����ҺF���ɹ���G ��
��4��Ҫ������C������E����G��ת��Ϊ��Ӧ���ȶ����������е�ʵ�����Ϊ ��
��5������������Һ����ı仯���������ҺH��c��Mg2����= ��