��Ŀ����
�������ƣ�NaClO2����һ����Ҫ�ĺ�������������Ҫ����ˮ�������Լ�ɰ�ǡ���֬��Ư����ɱ���������ǹ������ⷨ�����������ƵĹ�������ͼ��
��֪����NaClO2���ܽ�����¶����߶������ʵ������¿ɽᾧ����NaClO2?3H2O��
�ڴ�ClO2�ֽⱬը��һ����ϡ����������ϡ�͵�10�����°�ȫ��
��160 g/L NaOH��Һ��ָ160 gNaOH��������ˮ������Һ�����Ϊ1L��
��1��160 g/L NaOH��Һ�����ʵ���Ũ��Ϊ ����Ҫ�������Һ����������������Ҫ��һ�������� ��������˵������
��2���������й�����������ÿ����� ��ѡ����ţ���
a����SO2������SO3����ǿ���ԣ� b��ϡ��ClO2�Է�ֹ��ը��
c����NaClO3������ClO2
��3���������ڵķ�Ӧ�����ӷ�Ӧ����ʽΪ ��
���������¶Ȳ��ܳ���20�棬��Ŀ���� ��
��4���ڼ�����Һ��NaClO2�Ƚ��ȶ���������������Ӧά��NaOH�Թ������ж�NaOH�Ƿ�����ļ�ʵ�鷽���� ��
��5����������Ϊ��ֹNaClO2����ԭ��NaCl�����û�ԭ���Ļ�ԭ��Ӧ���С���H2O2�⣬������ѡ��Ļ�ԭ���� ��ѡ����ţ���
a��Na2O2 b��Na2S c��FeCl2
��6������Һ�еõ�NaClO2?3H2O�־����ʵ����������� ��ѡ����ţ�
a������ b������ c������ d������ e����ȴ�ᾧ
Ҫ�õ�������NaClO2?3H2O���������еIJ����� ����������ƣ�
��1��4 mol/L ��Һ���ܶȣ�2��b
��3��2OH-+2ClO2+H2O2��2ClO2-+O2��+2H2O ��ֹH2O2���ȷֽ⣬������NaClO2?3H2O������
��4�������ⶨ����������Һ��pHֵ ��5�� a��6��bed �ؽᾧ
���������������1������Ϣ��֪��160g/LNaOH��Һ��ʾ1L����������Һ����160gNaOH���������ʵ���Ũ�ȵĶ���ʽc=n/v����ɵø���Һ�������Ƶ����ʵ���Ũ��Ϊ4mol/L���������ʵ���Ũ�Ⱥ����������Ļ��㹫ʽ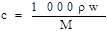 ��֪��Ҫ����������������Ҫ֪����Һ���ܶȡ�
��֪��Ҫ����������������Ҫ֪����Һ���ܶȡ�
��2������Ϣ�ڿ�֪����ClO2�ֽⱬը��һ����ϡ����������ϡ�͵�10%���°�ȫ���������й������������Ӧ��ϡ��ClO2�Է�ֹ��ը��
��3�����ݹ�������ͼ��֪���������ڵķ�Ӧ��ΪClO2��H2O2��NaOH��Һ����������NaClO2������һ����ClO2ת��ΪNaClO2�����ϼ۽��ͣ�����ԭ����H2O2�ض�����������������������Ӧ�����ӷ���ʽΪ2OH-+2ClO2+H2O2��2ClO2-+O2��+2H2O��H2O2���ȶ����¶ȹ������ֽ⣬���������¶Ȳ��ܳ���20�棬��Ŀ���Ƿ�ֹH2O2�ֽ⡣�����Ϣ��NaClO2���ܽ�����¶����߶��������������¶Ȳ��ܳ���20���ԭ��������NaClO2?3H2O��������
��4��NaOH��������Һ�ʼ��ԣ��ⶨ��Һ�����������ǣ������ⶨ����������Һ��pH��
��5����������ClO2ת��ΪNaClO2�Ļ�ԭ��ΪH2O2��Na2O2����ˮ�൱��H2O2����Na2S��FeCl2��ԭ�Խ�ǿ��ѡa��
��6������Һ�еõ����ᾧˮ�ľ����ȡ������Ũ������ȴ�ᾧ������ͨ�����˵õ��־��壮���Բ���˳��Ϊbed���õ��Ĵ־��徭���ؽᾧ�ɵõ����ȸ��ߵľ��塣
���㣺���������������Ʊ��Ļ�ѧ��������Ϊ���壬����ѧ���Ķ���Ŀ��ȡ��Ϣ�����������ʵ���Ũ�ȼ��㡢������ԭ��Ӧ���֪ʶ�ͻ�ѧ����ʵ�������

 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д����и������ʵķ�����ᴿ��Ӧѡ��������������һ�֣�����ѡ����ĸ��
| A����Һ | B������ | C����ȡ | D������E�������ᾧ����F�����·ֽ� |
�ڳ�ȥ����ʯ��ˮ��������CaCO3������ �� ������
�۳�ȥCaO������������CaCO3���壺���� �� ������
�ܴӵ�ˮ����ȡ�⣺���� �� ������
�ݷ���CCl4���е�Ϊ76.75�棩�ͼױ����е�Ϊ110.6�棩��Һ��������� �� ������
��ȥ���������������������ʣ���д��ѡ�õ��Լ��ͷ��뷽��
| | ����� (������Ϊ��������) | �Լ� �������� | ���뷽�� |
| A | �������ӣ� | | |
| B | ��ϩ��SO2�� | | |
| C | �������������ᣩ | | |
| D | �Ҵ���ˮ�� | | |
 Cr2O3��ұ��������Ҫ�������£�
Cr2O3��ұ��������Ҫ�������£�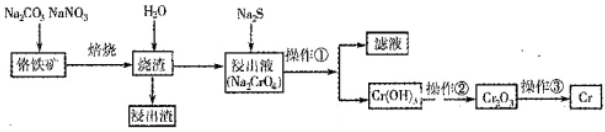
 4Na2CrO4��Fe2O3��4CO2��______________��
4Na2CrO4��Fe2O3��4CO2��______________��
 �����������[ ��NH4��2Fe��SO4��
�����������[ ��NH4��2Fe��SO4��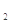 ]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3����
]�ζ����յ�ʱ����b mL���˲����Ŀ������Fe2���Ѷ����Cr2O72��ת��Ϊ��Cr3����
 ZnFe2(C2O4)3��6H2O������������(a)
ZnFe2(C2O4)3��6H2O������������(a) ZnFe2O4+2CO2��+4CO��+6H2O ����������(b)
ZnFe2O4+2CO2��+4CO��+6H2O ����������(b)

 3NH3 + 8AlO2��
3NH3 + 8AlO2��