��Ŀ����
��Ԫ�أ�Ce������ϵ��������Ȼ�����ߵ�һ�֣�������̬��+3��+4����ĺϽ����£������������������ƽ��������

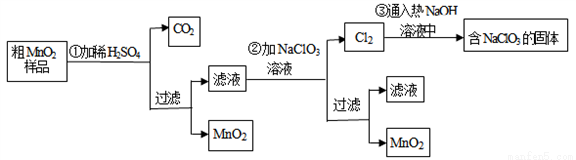
��1�������к��д�������Ⱦ��NO�����Ա�Ce4+��Һ���գ�����NO2����NO3�����������ʵ���֮��Ϊ1��1�����÷�Ӧ�������뻹ԭ�������ʵ���֮��Ϊ___________��
��2���ɲ��õ�ⷨ����������Һ�е�NO2��ת��Ϊ�����ʣ�ͬʱ����Ce4+����ԭ������ͼ��ʾ��
��Ce4+�ӵ��۵�__________������ĸ���)��������
��д�������ĵ缫��Ӧʽ____________________________��ÿ����1mol NO2����������H+���ʵ�������______mol��
��3����Ԫ������Ȼ����Ҫ�Է�̼����ʽ���ڣ���Ҫ��ѧ�ɷ�ΪCeFCO3����ҵ�����÷�̼�����ȡCeCl3��һ�ֹ����������£�
�ٱ��չ����з�������Ҫ��Ӧ����ʽΪ______________________________________��
�������������ͬѧ��Ϊ��ϡ�����H2O2�滻������ã�����������_________________________��
��Ce(BF4)3��KBF4��Ksp�ֱ�Ϊa��b����Ce(BF4)3(s) + 3KCl(aq) 3KBF4(s) + CeCl3 (aq)ƽ�ⳣ��Ϊ______________________��
3KBF4(s) + CeCl3 (aq)ƽ�ⳣ��Ϊ______________________��
�ܼ���CeCl3��6H2O��NH4Cl�Ĺ�������ɵõ���ˮCeCl3������NH4Cl��������______________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����ʵ���ܴﵽԤ��Ŀ�ĵ���
��� | ʵ������ | ʵ��Ŀ�� |
A | ��ij����Һ��ͨ��Cl2 ,�ٵ���2��KSCN��Һ����Һ��ΪѪ��ɫ | ֤���ô���Һ��һ������Fe2+ |
B | ��ij����Һ�м������ᣬ������ʹ����ʯ��ˮ����ǵ����� | ֤���ô���Һ��һ������CO32- |
C | �������ữ��H2O2��Һ����FeCl2��Һ�У���Һ��ɻ�ɫ | ֤��H2O2�����Դ���Fe3+ |
D | ��Al(0H)3�����зֱ��������Ͱ�ˮ�����������ܽ� | ֤��Al(0H)3�������������� |
A. A B. B C. C D. D
ҽ���Ȼ��ƿ������������ơ������������ȣ��Թ�ҵ̼��ƣ�������Na+��Al3+��Fe3+������)����ҽҩ����ˮ���Ȼ���(CaCl2��2H2O����������Ϊ97.0%��103.0%)����Ҫ�������£�

��֪��
�������� | Fe��OH��3 | Al��OH��3 | Al��OH��3 | |
��ʼ����ʱ��pH | 2.3 | 4.0 | ��ʼ�ܽ�ʱ��pH | 7.8 |
��ȫ����ʱ��pH | 3.7 | 5.2 | ��ȫ�ܽ�ʱ��pH | 10.8 |
��1��CaCO3�����ᷴӦ�����ӷ���ʽ___________��
��2�������ӡ������Ǽ����������ƣ�������Һ��pH��ΧΪ________��Ŀ���dz�ȥ��Һ�е�����Al3+��Fe2+��
��3������ʱ���õıȲ�������__________��
��4�����ữ�������Ǽ������ᣬ������Һ��pHԼΪ4.0����Ŀ���У��ٷ�ֹ�����������տ����еĶ�����̼���ڷ�ֹCa2+������ʱˮ�⣻��_______��
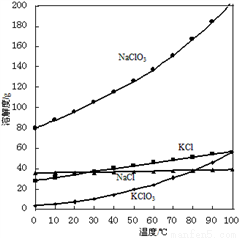
��5�������ᾧҪ������160���ԭ����__________��
��6���ⶨ��Ʒ��Cl-�����ķ����ǣ���ȡ0.750 0 gCaCl2��2H2O��Ʒ���ܽ⣬��250 mL����ƿ�ж��ݣ���ȡ25.00 mL������Һ����ƿ�У���0.050 00 mol/L AgNO3��Һ�ζ����յ㣨��K2Cr2O2��������AgNO3��Һ�����ƽ��ֵΪ20.39 mL��
�������ⶨ������������Һ��ϴ��������________��
�ڼ���������Ʒ��CaCl2��2H2O����������Ϊ_______����������λ��Ч���֣�
���������������ⶨ����Ʒ��CaCl2��2H2O����������ƫ��(�ⶨ�����в��������ɺ���)�������ԭ����________��__________��


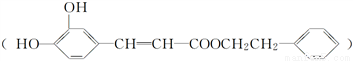 ��һ����Ȼ����ҩ���һ���������ܷ�������ת����
��һ����Ȼ����ҩ���һ���������ܷ�������ת����