��Ŀ����
��14�֣������(HF)��һԪ���ᣬ��ˮ��Һ�еĵ��뷽��ʽΪ��HF H����F����25���£���20mL0.2mol•L-1��������еμ�0.2mol•L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ��
H����F����25���£���20mL0.2mol•L-1��������еμ�0.2mol•L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ��

��֪������ȣ� ���͵���ƽ�ⳣ����K��һ�������������������������ϡ��Һ�еĵ���������
���͵���ƽ�ⳣ����K��һ�������������������������ϡ��Һ�еĵ��������� ��
��
��ش��������⣺
��1����������ϡ��Һ�У�ͨ���ı�����������ʹ�����ĵ����[ (HF)]�������_______����ʹ�����ĵ���ƽ�ⳣ��[Ka(HF)] �������_______��
(HF)]�������_______����ʹ�����ĵ���ƽ�ⳣ��[Ka(HF)] �������_______��
a�������¶� b������Һ�е���2��Ũ���� c����������NaF���� d����ˮϡ��
��2���ڴ��¶��£������ĵ���ƽ�ⳣ��Ka(HF)Ϊ��__
��������λ��Ч���֣�������� ԼΪ___
_%��
ԼΪ___
_%��
��3����֪�ڴ��¶��£�
H��(aq)��OH��(aq)��H2O(1) ��H����a kJ•mol -1��
HF(aq)  H��(aq)��F��(aq) ��H����b kJ•mol -1
H��(aq)��F��(aq) ��H����b kJ•mol -1
��ù����кͷ�Ӧ���Ȼ�ѧ����ʽΪ��________________________________ ���ں���F��ˮ�������£���Ӧ�ﵽB��ʱ���μӹ��������ͷŵ�����Ϊ________kJ����ֻ��a��b��ʽ�ӱ�ʾ����
��4�������й�ͼ�и�����Ũ�ȹ�ϵ��ȷ����_______________��
a����A����Һ�У�c(F��)+c(HF)=0.2 mol•L-1
b����B����Һ�У�c(H+)+c(HF)= c(OH��)
c����B����Һ�У�c(Na+)��c(F��)��c(H+)��c(OH��)
d����A��B����Һ�ж����ϣ� = Ka(HF)
= Ka(HF)
(1) a d��2�֣��� a��2�֣� (2) 5.3��10��4��2�֣��� 5��2�֣�
��3��HF(aq)+NaOH(aq)= NaF(aq)+ H2O(l)����H����b��a�� kJ•mol ��1��2�֣���0.004��a��0.95b����2�֣� (4) b d��2�֣�
����������1�����������ȵģ�����a�����������ȣ�Ҳ���������ƽ�ⳣ����b������������Ũ�ȣ����Ƶ��룬����ȼ�С��������ƽ�ⳣ�����䡣C�����������Ũ�ȣ����Ƶ��룬����ȼ�С��������ƽ�ⳣ�����䡣ϡ�ʹٽ����룬�������������ƽ�ⳣ�����䡣���Դ𰸷ֱ�ѡda��a��
��2��0.2mol•L-1���������Һ��������Ũ����0.01mol/L�����Ե������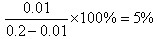 ������ƽ�ⳣ��Ϊ
������ƽ�ⳣ��Ϊ
��3�������˹���ɵ�Ӧ�á���ʽ��Ӽ��õ�HF(aq)+NaOH(aq)= NaF(aq)+ H2O(l)�����Է�Ӧ�ȡ�H����b��a�� kJ•mol ��1����Ӧ�ﵽB��ʱ������ǡ�÷�Ӧ������0.004molˮ��0.004molNaF�������ٷ�Ӧ��֮ǰ��HF�Ѿ���5���ķ����˵��룬����ʵ�ʷų���������0.004��a��0.95b��kJ��
��4��a����ȷ����Ϊ��Һ������Ѿ�����20ml�ˡ�B��ȷ�����������غ㡣B�����ڷ�����ˮ���Լ��ԣ�c����ȷ��D��ȷ�����ϵ���ƽ�ⳣ���ı���ʽ����ѡbd��

 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д� H����F����25���£���20mL0.2mol?L-1��������еμ�0.2mol?L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ��
H����F����25���£���20mL0.2mol?L-1��������еμ�0.2mol?L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ��
 ���͵���ƽ�ⳣ����K��һ�������������������������ϡ��Һ�еĵ���������
���͵���ƽ�ⳣ����K��һ�������������������������ϡ��Һ�еĵ��������� ��
��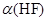 ԼΪ___ _%��
ԼΪ___ _%�� = Ka(HF)
= Ka(HF)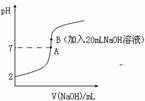 ��֪������ȣ�
��֪������ȣ�