��Ŀ����
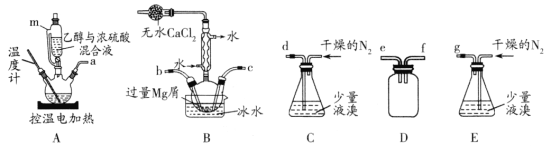
����Ŀ����ˮMgBr2�����ڴ�����ij��ѧС����ʵ����ѡ����ͼ��ʾװ�� (�г�װ���ԣ�����þм��Һ��Ϊԭ���Ʊ���ˮMgBr2��
��֪������Ũ��������£����ȵ�140��ʱ�Ҵ���ˮ��������(C2H5OC2H5)�����ȵ�170��ʱ�Ҵ���ˮ����CH2 = CH2��
�����ѵ��۵�Ϊ34.6�棬�е�Ϊ132�档
��Mg��Br2���ҷ�Ӧ���ų��������ȣ�MgBr2����ǿ��ˮ�ԣ�MgBr2�������ѷ�����Ӧ MgBr2 +3C2H5OC2H5 ![]() MgBr2 3C2H5OC2H5��
MgBr2 3C2H5OC2H5��
ʵ����Ҫ�������£�
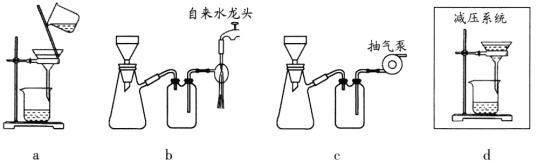
I.ѡ����������װ�ã���ȷ���ӣ����װ�õ������ԡ���װ���м���ҩƷ��
II.����װ��A��Ѹ��������140�棬������140�����һ��ʱ�䣬ֹͣ���ȡ�
III.ͨ�����ĵ�������Һ�建������װ��B�У�ֱ����ȫ���롣
IV.װ��B�з�Ӧ��Ϻ�ָ������£����˷�Ӧ����õ�����Һת�����������ƿ�У��ڱ�ˮ����ȴ���������壬�ٹ��˵������Ѻ��廯þ�ֲ�Ʒ��
V.�ñ�ϴ�Ӵֲ�Ʒ����ѹ���ˣ��������Ѻ��廯þ�����������160��ֽ����ˮ MgBr2��
�ش��������⣺
(1)װ��A��ʹ������m���ŵ���________��
(2)����I����ѡװ�õ���ȷ����˳����a________ (��Сд��ĸ)��װ��D��������________��
(3)������װ��Aһ��ʱ��������Ǽ������Ƭ��Ӧ�ò�ȡ����ȷ������________��
(4)����V���ñ�ϴ�������Ѻ��廯þ��Ŀ����________��
(5)����V���ü�ѹ���ˣ�ʹ������ѹǿ���ͣ��Դﵽ��Һ���ٷ��룩������װ�ÿ�������ѹ���˵���________(����ţ���
(6)ʵ�������¶ȿ��Ʋ�����װ��B�л����CH2Br��CH2Br�������ʵ����֤ CH2Br��CH2Br�Ĵ��ڣ��ӷ�Ӧ��Ļ�����з����ᴿ�õ�CH2Br��CH2Br��_______��
���𰸡�ʹϵͳ��ѹǿ��ȣ�����Һ��˳������ efbcg��ef�ɵߵ���bc�ɵߵ��� ��ֹ���� ֹͣ���ȣ���ȴ�����Ƭ ��ȥ���Ѻ��Ҵ� bc ȡ����CH2Br-CH2Br���Թ��У�����NaOH��Һ�����ȣ��ټ���ϡ�����ữ���μ�AgNO3��Һ���е���ɫ�������ɣ�֤����CH2Br-CH2Br��
��������
��ʵ��Ŀ�ĺ�ʵ�鲽�迴����þм��Һ���Ϊԭ�����Ʊ������Ѻ��廯þ���ټ��ȷֽ��Ʊ���ˮMgBr2������Aװ���Ʊ����Ѳ�����װ��B���м��װ��D��ֹ��������װ��E����������������װ��B��ʹ����þ��Ӧ��
(1)װ��A������mΪ��ѹ©��������ർ�ܿ�ʹ©���ں���ƿ��������ͨ��ѹǿ��ȣ���ѹ©��ʱҺ����˳�����¡�
(2)Ϊʹþ���塢���ѷ�Ӧ����MgBr2 3C2H5OC2H5����װ��A�Ʊ����Ѳ�����װ��B����װ��D��ֹװ��B��Һ�嵹����װ��A�У�װ��E�ø���ĵ�������������������װ��B������������˳��Ϊa��e��f��b��c��g��ef�ɵߵ���bc�ɵߵ�����
(3)����Һ���������������������Ƭ��ֹ���С�������װ��Aһ��ʱ��������Ǽ������Ƭ��Ӧ��ֹͣ���ȣ���ȴ�����Ƭ��
(4)����IV�õ��������Ѻ��廯þ�ֲ�Ʒ���������Ѻ��Ҵ�������V���ñ�ϴ�Ӿ���Ϊ�˳�ȥ��Щ���ʡ�
(5)��ѹ������ʹ������ѹǿ���Ͷ�������ѹǿ���䣬�Կ��ٷ����Һ����װ�� b��ˮ�����װ���ڿ�����װ��c�ó����ó��װ���ڿ�����ʹװ����ѹǿ��С��װ��a��d������ʹ��Һ���������ѹǿ�����ܼ��ٹ�Һ���롣
(6)��װ��A�¶ȿ��Ʋ�������������CH2=CH2���壬��װ��B������ӳɲ���CH2Br��CH2Br������CH2Br��CH2Br��������ԭ�ӣ��ɽ���ˮ��Ϊ�����ӣ�����ϡ���ᡢAgNO3��Һ���������ӣ��Ӷ�֤������ڡ��������Ϊ���ӷ�Ӧ��Ļ�����з����ᴿ�õ�CH2Br��CH2Br��ȡ����CH2Br-CH2Br���Թ��У�����NaOH��Һ�����ȣ��ټ���ϡ�����ữ���μ�AgNO3��Һ���е���ɫ�������ɣ�֤����CH2Br-CH2Br��

����Ŀ����X��Y��Z��T��U���ֶ�����Ԫ�ء�X��Y��Z ��Ԫ�������ڱ��е�λ����ͼ��ʾ����Ԫ�ص�ԭ������֮���� 41��X �� T �ĵ����ڲ�ͬ�����·�Ӧ����������T2X(��ɫ����)��T2X2(����ɫ����)���ֻ����U ������ Z ������ȼ��ʱ������ɫ���棬�������ˮ��Һ��ʹʯ����Һ��졣
X | |
Y | Z |
(1)��Ԫ�صķ����� X______��Y______��Z______��
(2)Y ԭ�ӵĽṹʾ��ͼΪ_______��
(3)�õ���ʽ��ʾ Y �� T ��ɵĻ�������γɹ��̣�______��
(4)YX2 �� U2Y ��Ӧ�Ļ�ѧ����ʽΪ______�� ������������______����������Ԫ����______��