��Ŀ����
��֪��N2 (g) +3H2(g)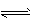 2NH3(g) ��H = -92.4 kJ . mol-1��
2NH3(g) ��H = -92.4 kJ . mol-1��
(1)��500�桢2.02��107Pa�����������£���2L�ܱ������г���1 mol N2��3 mol H2��10 min ʱ�ﵽƽ�⣬��0.2 mol NH3���ɣ���10 min���õ�����ʾ�ĸ÷�Ӧ��ƽ������v(N2)Ϊ ___��H2��ת����Ϊ___��
(2)��ַ�Ӧ�ų�������___���<������>����=����92.4 kJ��������___��
(3)Ϊ��Ч���������ת���ʣ�ʵ���������˲�ȡ�Ĵ�ʩ��__��
A�������¶�
B�����ϲ��䵪��
C�������³���He������ѹǿ
D�������¶�
E��ԭ��������ѭ��
F����ʱ�Ƴ���
(4)����������NO�����ȵIJ���Ͻ�������½��еģ���ӦΪ4NH3(g)+5O2(g)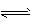 4NO(g)+6H2O(g)����Ӧ�����кϽ�ʼ�ձ��ֺ��ȣ��÷�Ӧ�Ļ�ѧƽ�ⳣ��K�ı���ʽΪ ____���������¶�ʱ��Kֵ___�����������С�����䡱����NH3��ת�������¶�T1���淴Ӧʱ��(t)�ı仯��ͼ��ʾ�������������䣬���ı��¶�ΪT2��T2����T1�����ڿ�ͼ�����������¶�T2��NH3��ת�����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��
4NO(g)+6H2O(g)����Ӧ�����кϽ�ʼ�ձ��ֺ��ȣ��÷�Ӧ�Ļ�ѧƽ�ⳣ��K�ı���ʽΪ ____���������¶�ʱ��Kֵ___�����������С�����䡱����NH3��ת�������¶�T1���淴Ӧʱ��(t)�ı仯��ͼ��ʾ�������������䣬���ı��¶�ΪT2��T2����T1�����ڿ�ͼ�����������¶�T2��NH3��ת�����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��
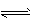 2NH3(g) ��H = -92.4 kJ . mol-1��
2NH3(g) ��H = -92.4 kJ . mol-1�� (1)��500�桢2.02��107Pa�����������£���2L�ܱ������г���1 mol N2��3 mol H2��10 min ʱ�ﵽƽ�⣬��0.2 mol NH3���ɣ���10 min���õ�����ʾ�ĸ÷�Ӧ��ƽ������v(N2)Ϊ ___��H2��ת����Ϊ___��
(2)��ַ�Ӧ�ų�������___���<������>����=����92.4 kJ��������___��
(3)Ϊ��Ч���������ת���ʣ�ʵ���������˲�ȡ�Ĵ�ʩ��__��
A�������¶�
B�����ϲ��䵪��
C�������³���He������ѹǿ
D�������¶�
E��ԭ��������ѭ��
F����ʱ�Ƴ���
(4)����������NO�����ȵIJ���Ͻ�������½��еģ���ӦΪ4NH3(g)+5O2(g)
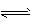 4NO(g)+6H2O(g)����Ӧ�����кϽ�ʼ�ձ��ֺ��ȣ��÷�Ӧ�Ļ�ѧƽ�ⳣ��K�ı���ʽΪ ____���������¶�ʱ��Kֵ___�����������С�����䡱����NH3��ת�������¶�T1���淴Ӧʱ��(t)�ı仯��ͼ��ʾ�������������䣬���ı��¶�ΪT2��T2����T1�����ڿ�ͼ�����������¶�T2��NH3��ת�����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ��
4NO(g)+6H2O(g)����Ӧ�����кϽ�ʼ�ձ��ֺ��ȣ��÷�Ӧ�Ļ�ѧƽ�ⳣ��K�ı���ʽΪ ____���������¶�ʱ��Kֵ___�����������С�����䡱����NH3��ת�������¶�T1���淴Ӧʱ��(t)�ı仯��ͼ��ʾ�������������䣬���ı��¶�ΪT2��T2����T1�����ڿ�ͼ�����������¶�T2��NH3��ת�����淴Ӧʱ��仯��Ԥ�ڽ��ʾ��ͼ�� 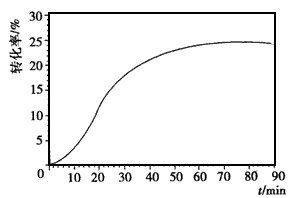
(1)0.005 mol��L-1��min-1��10%
(2)<����101 kPa��298 K�����£�1 mol������3 mol ������ȫ��Ӧ����2 mol�������ų�92.4 kJ�������÷�ӦΪ���淴Ӧ����Ӧ�ﲻ��ȫ��ת��Ϊ���������Ϊ��Ӧ�¶�Ϊ 500�棬���Էų�������С��92.4 kJ
(3)BEF
(4)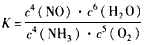 ������
������

(2)<����101 kPa��298 K�����£�1 mol������3 mol ������ȫ��Ӧ����2 mol�������ų�92.4 kJ�������÷�ӦΪ���淴Ӧ����Ӧ�ﲻ��ȫ��ת��Ϊ���������Ϊ��Ӧ�¶�Ϊ 500�棬���Էų�������С��92.4 kJ
(3)BEF
(4)
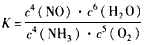 ������
������

��ϰ��ϵ�д�
 ���Ž�������С״Ԫϵ�д�
���Ž�������С״Ԫϵ�д�
�����Ŀ