��Ŀ����
13���Իش������к��Ȳⶨ���й�������1��ʵ�����ϱ����ձ�����С�����ձ�������ĭ���ϡ���ĭ���ϰ塢���β����������0.50mol•L-1���ᡢ0.55mol•L-1NaOH��Һ����ȱ�ٵ�ʵ����Ʒ����Ͳ���¶ȼƣ�
��2��ʵ�����ܷ��û���ͭ˿��������滷�β������������ܡ����ܡ������ܣ���ԭ���ǽ������ȣ�����ɢʧ��������
��3��ʵ��ʱ�������ἰNaOH��Һ�������Ϊ50mL������Һ�ܶ�Ϊ1g/cm3��������Һ�ı�����c=4.18J/��g•�棩��ʵ����ʼ�¶�Ϊ20�棬��ֹ�¶�Ϊ23.2�森�Լ���÷�Ӧ���к��ȡ�H=-53.5kJ•mol-1��
���� ��1�������к��Ȳⶨ��ʵ�鲽��ѡ����Ҫ��������Ȼ���жϻ�ȱ�ٵ�������
��2���������ȿ죬������ʧ�ࣻ
��3�����ݹ�ʽQ=cm��T���������0.025mol��ˮ�ų��������������к��ȵĸ�������к��ȣ�
��� �⣺��1���к��ȵIJⶨ�����У���Ҫ����Ͳ��ȡ����Һ������Һ���������Ҫʹ���¶ȼƲ����¶ȣ����Ի�ȱ���¶ȼƺ���Ͳ��
�ʴ�Ϊ����Ͳ���¶ȼƣ�
��2�������û���ͭ˿��������滷�β������������Ϊͭ˿��������ȵ������壬������ʧ��
�ʴ�Ϊ�����ܣ��������ȣ�����ɢʧ��������
��3��50mL0.50mol•L-1������50mL0.55mol•L-1NaOH��Һ��ϣ��������ƹ�������Ӧ������0.025molˮ����Ӧǰ���¶Ȳ�Ϊ��3.2�棻50mL0.50mol•L-1������50mL0.55mol•L-1NaOH��Һ��������Ϊ��m=100mL��1g/mL=100g��c=4.18J/��g•�棩�����빫ʽQ=cm��T������0.025mol��ˮ�ų�����Q=4.18J/��g•�棩��100g��3.2��=1.3376kJ��������0.025mol��ˮ�ų�����1.3376kJ����������1mol��ˮ�ų�����Ϊ1.3376kJ��$\frac{1mol}{0.025mol}$=-53.5kJ������ʵ���õ��к��ȡ�H=-53.5kJ•mol-1��
�ʴ�Ϊ��-53.5kJ•mol-1��
���� ���⿼�������к��ȵIJⶨ��������Ӧ�ȵļ��㣬��Ŀ�Ѷ��еȣ�ע�������к��Ȳⶨԭ��������������ѧ�����Ӧ����ѧ֪ʶ��������

 Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�| A�� | O | B�� | P | C�� | Si | D�� | Fe3+ |
| A�� | ������Ԫ�����ڱ��еڶ����ڢ�A�� | |
| B�� | �����������Ϊ+5������Ϊ-3 | |
| C�� | ��������������Ӧˮ������һ��ǿ�� | |
| D�� | ���ķǽ����Ա���������ǿ |
| A�� | NaOH H2SO4 ��NH4��2SO4 | B�� | MgO Na2SO4 HNO3 | ||
| C�� | Na2O2 KOH Na3PO4 | D�� | HCl Al2O3 MgCl2 |
| A�� | 1.0 mol•L-1��KNO3��Һ��H+��Fe2+��Cl-��SO42- | |
| B�� | ʹʯ��ʺ�ɫ����Һ��NH4+��Ba2+��ClO-��Cl- | |
| C�� | ʹ��̪�ʺ�ɫ����Һ��K+��Na+��CH3COO-��Br- | |
| D�� | ������Ӧ����������������Һ��Na+��K+��CO32-��NO3- |
| A�� | CH2CH2--��ϩ�Ľṹ��ʽ | B�� | �Ҵ��Ľṹ��ʽ--C2H6O | ||
| C�� | 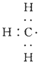 --���ĵ���ʽ --���ĵ���ʽ | D�� | ���Ȼ�̼�ĵ���ʽΪ--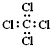 |
| A�� | �屽��Һ�壩�������Ȼ�̼����ȡ | |
| B�� | ���飨��ϩ����ͨ����������Һ��ϴ�� | |
| C�� | �����飨�Ҵ�������ˮ����Һ | |
| D�� | �������������ᣩ��������������Һ����Һ |
| A�� | ��AgNO3����NaBr����AgBr | B�� | ��AgNO3����NaCl����AgCl | ||
| C�� | ��AgCl�� ��AgNO3����NaCl | D�� | ��AgNO3����NaCl����Ag |
| A�� | ��̬�⻯����ȶ���X��Y��Z��˳����� | |
| B�� | ����Ԫ�ص�����������Ӧ��ˮ�������ԣ�H2ZO3��H3YO4��H2XO4 | |
| C�� | Ԫ�ص���������ϼ۰�X��Y��Z��˳����� | |
| D�� | Ԫ��ԭ�ӵİ뾶��X��Y��Z��˳����� |