��Ŀ����
ij����С������ͼ��ʾ��ʵ��װ��̽�������백��֮��ķ�Ӧ������A��FΪ�����Ͱ����ķ���װ�ã�DΪ����������������백����Ӧ��װ�á�

��ش���������
��1��ʵ�����ö������̺�Ũ���ᷴӦ��ȡ������ͬʱ���ɶ��Ȼ��̺�ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ
________________________
��2��ϴ��ƿB��������_______________��C��E���Ǹ���װ�ã�����E�и��������װ���Լ���________
��3����Ӧ��ʼ��װ��D�г���Ũ��İ��̲��������ڱ����ᣬ�С�������ͨ��ʵ������ù���ijɷ֡�����Э��������ɸ�ʵ��̽����
����٣�ȡ������Ʒ������ˮ�ܽ⣬��װ����֧�Թ��С�������һ֧�Թ��м���ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڸ�������ɫʯ����ֽ������֤���ù����к���________________
����ڣ�________________________
��4������ɫ��ѧ�ĽǶȿ��Ǹ�װ������һ���Բ�����֮������������Ľ��������
________________________________
��1��ʵ�����ö������̺�Ũ���ᷴӦ��ȡ������ͬʱ���ɶ��Ȼ��̺�ˮ��д���÷�Ӧ�Ļ�ѧ����ʽ
________________________
��2��ϴ��ƿB��������_______________��C��E���Ǹ���װ�ã�����E�и��������װ���Լ���________
��3����Ӧ��ʼ��װ��D�г���Ũ��İ��̲��������ڱ����ᣬ�С�������ͨ��ʵ������ù���ijɷ֡�����Э��������ɸ�ʵ��̽����
����٣�ȡ������Ʒ������ˮ�ܽ⣬��װ����֧�Թ��С�������һ֧�Թ��м���ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڸ�������ɫʯ����ֽ������֤���ù����к���________________
����ڣ�________________________
��4������ɫ��ѧ�ĽǶȿ��Ǹ�װ������һ���Բ�����֮������������Ľ��������
________________________________
��1��4HCl+MnO2 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
��2����ȥCl2�е�HCl����ʯ��
��3������٣�NH4+ ������ڣ�����һ֧�Թ��м��������ữ����������Һ����������ɫ������˵����Һ�к���Cl-����
��4����Dװ�õ���ֱ���ܿ���һ�������ռ�β�����Է�β����Ⱦ����
 MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O��2����ȥCl2�е�HCl����ʯ��
��3������٣�NH4+ ������ڣ�����һ֧�Թ��м��������ữ����������Һ����������ɫ������˵����Һ�к���Cl-����
��4����Dװ�õ���ֱ���ܿ���һ�������ռ�β�����Է�β����Ⱦ����

��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д� �Ƹ������������ϵ�д�
�Ƹ������������ϵ�д�
�����Ŀ

 ij����С������ͼ��ʾװ�ý���ʵ�飨���Һ������������˵����ȷ���ǣ�������
ij����С������ͼ��ʾװ�ý���ʵ�飨���Һ������������˵����ȷ���ǣ�������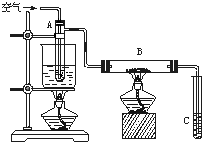 ij����С������ͼ��ʾ�����Ҵ��Ĵ�����ʵ��̽�����Թ�A��ʢ����ˮ�Ҵ���B��װ��CuO����ʯ���������壩���Թ�C�зŵ�������ˮ����ش��������⣺
ij����С������ͼ��ʾ�����Ҵ��Ĵ�����ʵ��̽�����Թ�A��ʢ����ˮ�Ҵ���B��װ��CuO����ʯ���������壩���Թ�C�зŵ�������ˮ����ش��������⣺

