��Ŀ����
��10�֣��л���A������ʳƷ��ҵ����֪9.0 g A������O2�г��ȼ�գ������ɵĻ����������ͨ��������Ũ����ͼ�ʯ�ң��ֱ�����5.4 g��13.2 g��������ʣ������ΪO2��
��1��A���ӵ�����ͼ����ͼ��ʾ����ͼ�п�֪�����
����������90����A�ķ���ʽ��______________________��

��2��A����NaHCO3��Һ������Ӧ��Aһ�����еĹ�����������______________��
��3��A���ӵĺ˴Ź���������4���壬�����֮����1��1��1��3����A�Ľṹ��ʽ��_________________________________��
��4��0.1 mol A������Na��Ӧ���ڱ�״���²���H2�������__________L��
��5��A��һ�������¿ɾۺϵõ�һ�־���������������������ߣ��䷴Ӧ�Ļ�ѧ����ʽ��___________________________��
��1��A���ӵ�����ͼ����ͼ��ʾ����ͼ�п�֪�����
����������90����A�ķ���ʽ��______________________��

��2��A����NaHCO3��Һ������Ӧ��Aһ�����еĹ�����������______________��
��3��A���ӵĺ˴Ź���������4���壬�����֮����1��1��1��3����A�Ľṹ��ʽ��_________________________________��
��4��0.1 mol A������Na��Ӧ���ڱ�״���²���H2�������__________L��
��5��A��һ�������¿ɾۺϵõ�һ�־���������������������ߣ��䷴Ӧ�Ļ�ѧ����ʽ��___________________________��
��10�֣�ÿ��2�֣�
��1��C3H6O3 ��2���Ȼ���3�� ��4��2.24
��4��2.24
��5��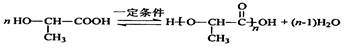
��1��C3H6O3 ��2���Ȼ���3��
 ��4��2.24
��4��2.24��5��
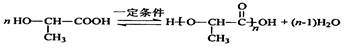
��1��Ũ������ˮ���������ɵ�ˮ��5.4g�����ʵ�����0.3mol����ʯ�����յ���CO2����CO2��13.2g�����ʵ�����0.3mol������9.0gA����ԭ�ӵ����ʵ�����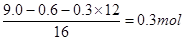 ��������ʽΪCH2O������Ϊ��Է���������90�����Է���ʽΪC3H6O3 ��
��������ʽΪCH2O������Ϊ��Է���������90�����Է���ʽΪC3H6O3 ��
��2��A����NaHCO3��Һ������Ӧ����Aһ�����еĹ��������Ȼ���
��3��������ԭ�ӵ����༰����֮�ȿ�֪���ṹ��ʽΪ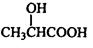 ��
��
��4��A�к���1���ǻ���1���Ȼ�������0.1mol������0.1mol��������״���µ������2.24L��
��5��A�к���1���ǻ���1���Ȼ���������ͨ��������Ӧ���ɸ߷��ӻ��������ʽΪ
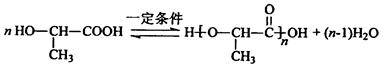
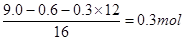 ��������ʽΪCH2O������Ϊ��Է���������90�����Է���ʽΪC3H6O3 ��
��������ʽΪCH2O������Ϊ��Է���������90�����Է���ʽΪC3H6O3 ����2��A����NaHCO3��Һ������Ӧ����Aһ�����еĹ��������Ȼ���
��3��������ԭ�ӵ����༰����֮�ȿ�֪���ṹ��ʽΪ
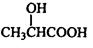 ��
����4��A�к���1���ǻ���1���Ȼ�������0.1mol������0.1mol��������״���µ������2.24L��
��5��A�к���1���ǻ���1���Ȼ���������ͨ��������Ӧ���ɸ߷��ӻ��������ʽΪ
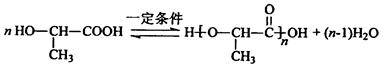

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 ��ʽ������ʾ�����ߵĽ�����˵㴦����̼ԭ�ӣ�������ԭ��������4�ۣ���C��Hԭ��δ��dz�����ά����A�ķ���ʽΪ_______________��1 molά����A�ڴ���������������_________ mol H2�����ӳɷ�Ӧ��
��ʽ������ʾ�����ߵĽ�����˵㴦����̼ԭ�ӣ�������ԭ��������4�ۣ���C��Hԭ��δ��dz�����ά����A�ķ���ʽΪ_______________��1 molά����A�ڴ���������������_________ mol H2�����ӳɷ�Ӧ��