��Ŀ����
����Ŀ������ԭ��ص������кܶ࣬����һ�־������õ���ʵ�Ũ�Ȳ�ɡ�Ũ���ء������������ij���ӵ�Ũ��Խ��ʱ�������Ի�ԭ��Խǿ����ͼ���׳�Ϊ3mol��L-1��AgNO3��Һ���ҳ�Ϊ1mol��L-1��AgNO3��Һ��A��B��ΪAg�缫��ʵ�鿪ʼ�ȱպ�K2���Ͽ�K1�����ֵ�����ָ�뷢��ƫת������˵������ȷ����

A. һ��ʱ��������ָ�뽫���㣬��ʱ����Ϊ��Ӧ���ٽ���
B. ��������ָ�����պ�K1���Ͽ�K2���ҳ���ҺŨ������
C. ��������ָ�����պ�K1���Ͽ�K2������Ag�缫��������
D. ʵ�鿪ʼ�ȱպ�K2���Ͽ�K1����ʱNO3-��B�缫�ƶ�
���𰸡�C
��������A. �պ�K2������Ũ���أ����ڼ׳���������Ũ�ȴ�������ǿ��������ԭ��Ӧ���׳�Ϊ�������ҳ�Ϊ�������׳�����������Ũ����С���ҳ�����������Ũ��������һ��ʱ���Ũ����ȣ����ٹ���Ũ���أ�������ָ�뽫���㣬��Ӧ���ٽ��У���A��ȷ��B. ��������ָ�����պ�K1���Ͽ�K2���ɵ��أ��ҳ�����Ϊ������������Ũ��������B��ȷ��C. ��������ָ�����պ�K1���Ͽ�K2���ɵ��أ��ҳ�����Ϊ���������缫���ܽ⣬������С����C����D. ʵ�鿪ʼ�ȱպ�K2���Ͽ�K1������Ũ���أ����ڼ׳���������Ũ�ȴ�������ǿ��������ԭ��Ӧ���׳�Ϊ�������ҳ�Ϊ�������׳�����������Ũ����С���ҳ�����������Ũ��������ʱNO3-��B�缫�ƶ�����D��ȷ����ѡC��

����Ŀ����������(NOCl)���л��ϳ��е���Ҫ�Լ�������NO��Cl2��Ӧ�õ�����ѧ����ʽΪ2NO(g)��Cl2(g) ![]() 2NOCl(g)��
2NOCl(g)��
��1�����������������ڴ����еĺ������������ʱ�������������ȣ��漰���·�Ӧ��
��2NO2(g)��NaCl(s) ![]() NaNO3(s)��NOCl(g)
NaNO3(s)��NOCl(g)
��4NO2(g)��2NaCl(s) ![]() 2NaNO3(s)��2NO(g)��Cl2(g)
2NaNO3(s)��2NO(g)��Cl2(g)
��2NO(g)��Cl2(g) ![]() 2NOCl(g)
2NOCl(g)
�跴Ӧ�٢ڢ۶�Ӧ��ƽ�ⳣ������ΪK1��K2��K3����K1��K2��K3֮��Ĺ�ϵΪ____________��
��2��300 ��ʱ��2NOCl(g) ![]() 2NO(g)��Cl2(g)������Ӧ���ʵı���ʽΪv����k��cn(NOCl)(kΪ���ʳ�����ֻ���¶��й�)�����������Ũ�ȵĹ�ϵ�����ʾ��
2NO(g)��Cl2(g)������Ӧ���ʵı���ʽΪv����k��cn(NOCl)(kΪ���ʳ�����ֻ���¶��й�)�����������Ũ�ȵĹ�ϵ�����ʾ��
��� | c(NOCl)/mol��L��1 | v/mol��L��1��s��1 |
�� | 0.30 | 3.60��10��9 |
�� | 0.60 | 1.44��10��8 |
�� | 0.90 | 3.24��10��8 |
n��________��k��________��
��3����1 L�����ܱ������г���2 mol NO(g)��1 mol Cl2(g)���ڲ�ͬ�¶��²��c(NOCl)��ʱ��t�Ĺ�ϵ��ͼA��ʾ����Ӧ��ʼ��10 minʱCl2��ƽ����Ӧ����v(Cl2)��________ mol��L��1��min��1��
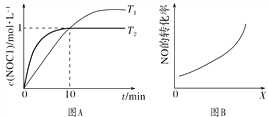
��4�����ܱ������г���NO(g)��Cl2(g)���ı��������[�¶ȡ�ѹǿ��![]() ��������ĽӴ����]��NO��ת���ʱ仯��ϵ��ͼB��ʾ��X����________��
��������ĽӴ����]��NO��ת���ʱ仯��ϵ��ͼB��ʾ��X����________��