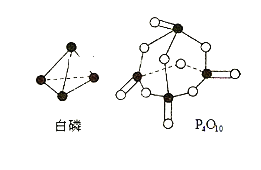
��Ŀ����
����Ŀ���й����ʵ����ļ�����գ�
��1��2 mol����[CO(NH2)2]��_____________��Hԭ�ӣ�������ԭ�Ӹ�__________g H2O������ԭ�Ӹ�����ȡ�
��2���ٱ�״���£�22.4 L CH4����1.5 mol NH3����1.806��1024��H2O���ܱ�״���£�73 g HCl������Hԭ�Ӹ����ɶൽ�ٵ�˳����____________________��
��3��30.9 g NaR����Na��0.3 mol����NaR��Ħ������Ϊ_________________��
��4��100mLijAl2(SO4)3��Һ�У�c(Al3��)��2.0 mol��L��1��������c(SO42��)��_________mol��L��1��
���𰸡�8��6.02��1023 36 ��>��>��>�� 103g/mol 3.0
��������
��1���ӻ�ѧʽ����2 mol����[CO(NH2)2]��8molHԭ�ӣ���2mol��ԭ�ӣ�����ԭ�Ӹ�����ȵ�H2OҲӦΪ2mol���ɴ˿������������
��2���ٱ�״���£�22.4 L CH4Ϊ1mol����4molHԭ�ӣ���1.5 mol NH3��4.5molHԭ�ӣ���1.806��1024��H2O��6molHԭ�ӣ��ܱ�״���£�73 g HClΪ2mol����2molHԭ�ӡ��ȽϿɵó�����Hԭ�Ӹ����ɶൽ�ٵ�˳��
��3��30.9 g NaR����Na��0.3 mol����NaRΪ0.3mol��Ħ������Ϊ![]() ��
��
��4��100mLijAl2(SO4)3��Һ�У�c(Al3��)��2.0 mol��L��1��������c(SO42��)��![]() mol��L��1��
mol��L��1��
��1���ӻ�ѧʽ����2 mol����[CO(NH2)2]��8molHԭ�ӣ������Ϊ8��6.02��1023����2mol��ԭ�ӣ�����ԭ�Ӹ�����ȵ�H2OҲӦΪ2mol��������Ϊ2mol��18g/mol=36g����Ϊ��8��6.02��1023��36��
��2���ٱ�״���£�22.4 L CH4Ϊ1mol����4molHԭ�ӣ���1.5 mol NH3��4.5molHԭ�ӣ���1.806��1024��H2O��6molHԭ�ӣ��ܱ�״���£�73 g HClΪ2mol����2molHԭ�ӡ��ȽϿɵó�����Hԭ�Ӹ����ɶൽ�ٵ�˳���>��>��>�ܡ���Ϊ����>��>��>�ܣ�
��3��30.9 g NaR����Na��0.3 mol����NaRΪ0.3mol��Ħ������Ϊ![]() ����Ϊ��103g/mol��
������103g/mol��
��4��100mLijAl2(SO4)3��Һ�У�c(Al3��)��2.0 mol��L��1��������c(SO42��)��![]() mol��L��1=3.0 mol��L��1����Ϊ��3.0��
mol��L��1=3.0 mol��L��1������3.0��

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д� ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�����Ŀ����ҵ�ϳ�����ұ��п�����е�п������ZnO��FeO��Fe2O3��CuO��Al2O3�����ʣ�������������Zn(NO3)2��6H2O���壬�乤������Ϊ��
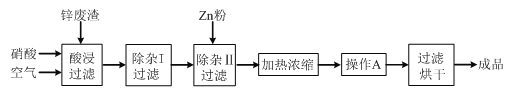
�й��������↑ʼ�����ͳ�����ȫ��pH���±���
�������� | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Cu(OH)2 | Zn(OH)2 |
��ʼ������pH | 3��3 | 1��5 | 6��5 | 4��2 | 5��4 |
������ȫ��pH | 5��2 | 3��7 | 9��7 | 6��7 | 8��0 |
���ڡ�����������У�Ϊ���п�Ľ������ʣ���ͨ����������衱�⣬���ɲ�ȡ�Ĵ�ʩ��_____________________��
���������������жദ�漰�����ˡ���ʵ�����й��˲�����Ҫʹ�õIJ���������_____��
���ڡ�����I�������У����ټ�������H2O2��Һ��H2O2��Fe2+��Ӧ�����ӷ���ʽΪ_____��ΪʹFe(OH)3��Al(OH)3������ȫ����Zn(OH)2��������Ӧ������Һ��pH��ΧΪ_____������Fe3+�Ƿ������ȫ��ʵ�������_____��
�ȼ���Zn�۵�������_____��������A����������_____��