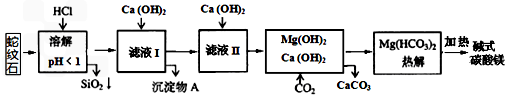
��Ŀ����
����ʯ��ɿ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�������ʯ�Ƽ�ʽ̼��þ��ʵ�鲽�����£� �����£���ؽ����������������KSP���±�1
|
B������Ksp��������ѧʽ���Ƶģ�KspԽС��Խ���γɳ�����
C����������������Ksp�������Al3+�պó�����ȫʱ��PH��
D��ȷ����ƷaMgCO3?bMg��OH��2?cH2O��a��b��c��ֵ����Ҫ�ⶨ�������Ǣ���Ʒ��������MgO������������CO2�����������������������ˮ��������
B������Ksp��������ѧʽ���Ƶģ�KspԽС��Խ���γɳ�������֪Fe��OH��3��KspС������Fe��OH��3���γɳ�������B����
C��Al3+�պó�����ȫʱc��Al3+��=1.0��10-5 mol?L-1����c��OH-��=
| 3 |
| ||
| 3 |
| ||
������PH=4.7����pH���ڵ�4.7ʱ��Al3+�պó�����ȫ����C��ȷ��
D��m����Ʒ��=18.2g��m��CO2��=6.6g��m��MgO��=8.0g����ʽ̼��þ�ֽ⣺aMgCO3?bMg��OH��2?cH2
| ||
��ѡB��

 ���Ӣ��������ϵ�д�
���Ӣ��������ϵ�д�����ʯ����Կ�����MgO��Fe2O3��Al2O3��SiO2��ɣ�������ʯ��ȡ��ʽ̼��þ��ʵ�鲽�����£�
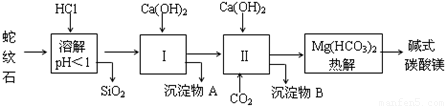
��1������ʯ��������ܽ����Һ�����Mg2+�⣬�����еĽ��������� ��
��2�����Т����ʱ��������Һ��pH=7~8���й��������������pH���±�����Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ�� �ܽ⣬���� ������
|
�������� |
Fe(OH)3 |
Al(OH)3 |
Mg(OH)2 |
|
��ʼ����pH |
1.5 |
3.3 |
9.4 |
��3���ӳ��������A����ȡ��ɫ��������Ϊ���ϣ����������A�м��� ����������ʵĻ�ѧʽ����Ȼ�� ��������дʵ��������ƣ�������ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ������� ����д���ʵĻ�ѧʽ����
��4�������ʵ�飬ȷ����ƷaMgCO3��bMg(OH)2��cH2O��a��b��c��ֵ����д������ʵ�鲽��������Ҫ�ⶨ����Ŀ�������Լ���Ũ���ᡢ��ʯ�ҡ�����������Һ������ʯ��ˮ��������Ʒ�������ڸ��·ֽ⣬�� ���� ����MgO������
��5��������������װ�б�Ҫ���Լ�����ѡ���������ʵ�������������������һ��װ�� ��ѡ���������ţ����ظ�ʹ�ã��á�A��B������������ʾ��
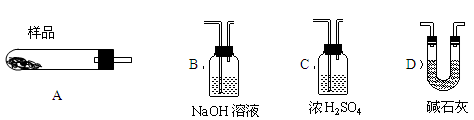
��6��18.2g��Ʒ��ȫ�ֽ����6.6gCO2��8.0gMgO���ɴ˿�֪����Ʒ�Ļ�ѧʽ��a= ��b= ��c= ��