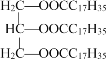��Ŀ����
����Ŀ����˾ƥ�֣�������L����������֪�Ľ�����ʹҩ�һ�ֳ�Ч�����Ͱ�˾ƥ�֣�������P����ṹ��ʽΪ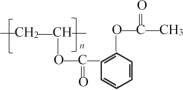 ���ĺϳ�·����ͼ��ʾ��
���ĺϳ�·����ͼ��ʾ��

��֪����HC![]() CH+RCOOH
CH+RCOOH![]()
��RCOOR��+R��OH![]() RCOOR��+R��OH(R��R����R����������)
RCOOR��+R��OH(R��R����R����������)
��1��A�еĹ�������___��
��2��C�Ľṹ��ʽ��___��
��3��D��E�ķ�Ӧ������___��
��4��E��G�Ļ�ѧ����ʽ___��
��5����֪��H�Ƿ����廯���L�����ڿɽϿ�ת��Ϊ����ҩЧ��J����������P��L��ȣ��������ܻ��������ͷ�J��
��ѪҺ��JŨ�ȹ�����ʹ���ж����ɾ�����עNaHCO3��Һ�ⶾ�����û�ѧ����ʽ����NaHCO3������___��
��д��J��L�Ļ�ѧ����ʽ___���˷�Ӧ�ĸ���Ӧ��J�����������ۣ�д��������Ľṹ��ʽ___��
���𰸡��ǻ� HC��CH �Ӿ۷�Ӧ 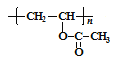 +nCH3CH2OH
+nCH3CH2OH![]() +n
+n![]()
![]() +NaHCO3
+NaHCO3![]() +H2O+CO2
+H2O+CO2 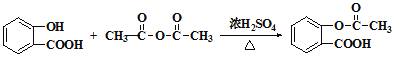 +CH3COOH
+CH3COOH 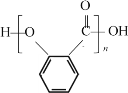
��������
����������P�Ľṹ��ʽ�� ��������ԭ��Ϊ�Ҵ��ͱ��ӣ���֪��Ҫ�����IJ�λ��ͼ��ʾ��
��������ԭ��Ϊ�Ҵ��ͱ��ӣ���֪��Ҫ�����IJ�λ��ͼ��ʾ��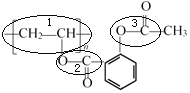 ������1��2��λ��������֪�١��ڵķ�Ӧ��ʽ���Ӿ۷�Ӧ��������Ӧ����������λ3�Ĺ����ɴӺϳ�·��ͼ�л�֪��
������1��2��λ��������֪�١��ڵķ�Ӧ��ʽ���Ӿ۷�Ӧ��������Ӧ����������λ3�Ĺ����ɴӺϳ�·��ͼ�л�֪��
�������Ϸ����������Ϣ��������P�ĺϳ�·��ӦΪ���Ƚ��Ҵ����������õ����ᣬ�ٰ���֪�ٵķ�Ӧ��ʽ����Ȳ��Ӧ����������ϩ��D��CH2=CHOOCCH3����һ�������Ӿ۷�Ӧ�õ�E��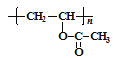 ��E����֪�ڵķ�Ӧ��ʽ���Ҵ���Ӧ�õ�G
��E����֪�ڵķ�Ӧ��ʽ���Ҵ���Ӧ�õ�G![]() ������������HΪ���ӣ�����I�ķ���ʽ�ͻ�����P�Ľṹ��֪����H��Ӧ���ɵ�I�ĽṹΪ��
������������HΪ���ӣ�����I�ķ���ʽ�ͻ�����P�Ľṹ��֪����H��Ӧ���ɵ�I�ĽṹΪ��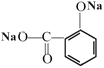 ���ữ����
���ữ����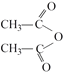 ��Ӧ����L
��Ӧ����L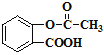 ��G��
��G��![]() ����L��
����L�� ������������Ӧ���ɻ�����P���������Ϸ�������л�֪ʶ�ɽ��С�⡣
������������Ӧ���ɻ�����P���������Ϸ�������л�֪ʶ�ɽ��С�⡣
��1��AΪ�Ҵ������������ǻ�����Ϊ���ǻ�
��2�����ݷ�����֪���Ҵ����������õ����ᣬ����C����֪�ٵķ�Ӧ��ʽ����CH2=CHOOCCH3�����ƶ�CΪHC��CH����Ϊ��HC��CH
��3��D��E�ķ�Ӧ��С����CH2=CHOOCCH3��һ�����������ɸ߷���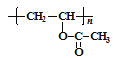 �����Ը÷�ӦΪ�Ӿ۷�Ӧ����Ϊ���Ӿ۷�Ӧ
�����Ը÷�ӦΪ�Ӿ۷�Ӧ����Ϊ���Ӿ۷�Ӧ
��4��������֪�ڣ�E��G�ķ�Ӧ��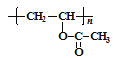 �ʹ���Ӧ�����µ������µĴ����Ӳ���F�ķ���ʽ��C4H8O2����֪��FΪ�����������ʸ÷�Ӧ�Ļ�ѧ����ʽΪ��
�ʹ���Ӧ�����µ������µĴ����Ӳ���F�ķ���ʽ��C4H8O2����֪��FΪ�����������ʸ÷�Ӧ�Ļ�ѧ����ʽΪ��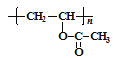 +nCH3CH2OH
+nCH3CH2OH![]() ������
������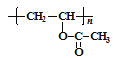 +nCH3CH2OH
+nCH3CH2OH![]() +n
+n![]()
��5����J�� �ữ��IJ���ṹΪ��
�ữ��IJ���ṹΪ��![]() ����������-COOH���Խ�ǿ����̼������ǿ������NaHCO3��Ӧ����������ǻ������Ա�̼������������NaHCO3��Ӧ������ѪҺ�е�J�þ�����עNaHCO3��Һ�ⶾ�Ļ�ѧ����ʽΪ��
����������-COOH���Խ�ǿ����̼������ǿ������NaHCO3��Ӧ����������ǻ������Ա�̼������������NaHCO3��Ӧ������ѪҺ�е�J�þ�����עNaHCO3��Һ�ⶾ�Ļ�ѧ����ʽΪ��![]() +NaHCO3
+NaHCO3![]() +H2O+CO2������
+H2O+CO2������![]() +NaHCO3
+NaHCO3![]() +H2O+CO2
+H2O+CO2
��JΪ![]() ��LΪ
��LΪ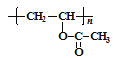 ������J��L�Ļ�ѧ����ʽΪ��
������J��L�Ļ�ѧ����ʽΪ��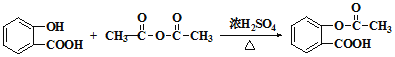 +CH3COOH��
+CH3COOH��
![]() ��˫�����ţ�-OH��-COOH������������������۷�Ӧ������
��˫�����ţ�-OH��-COOH������������������۷�Ӧ������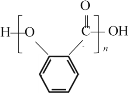 ����Ϊ��
������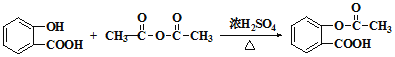 +CH3COOH��
+CH3COOH��

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�����Ŀ���±���A��B��X��D��E�����л�����й���Ϣ��
A | A�Ľṹ��ʽΪ |
B | ����ʹ������Ȼ�̼��Һ��ɫ �ڱ���ģ��Ϊ |
C | ����C��H����Ԫ����� �����ģ��Ϊ |
D | ����C��H��O����Ԫ����� ������Na��Ӧ����������NaOH��Һ��Ӧ |
E | ����C��H��O����Ԫ����� �����ģ��Ϊ |
��1��B��������Ȼ�̼��Һ��Ӧ���������������___��
��2��д����Ũ���������£�C��Ũ���ᷴӦ�Ļ�ѧ����ʽ___��
��3��A������NaOH��Һ���ȷ���������Ӧ�Ļ�ѧ����ʽ��___��
��4������BΪ��Ҫԭ�Ϻϳ����ᣬ��ϳ�·����ͼ��ʾ��
![]()
��F���ʹ����ŵ�������___��
��д����Ӧ�ڵĻ�ѧ����ʽ___����Ӧ����Ϊ___��
�۹�ҵ����BΪԭ�Ͽ��Ժϳ�һ���л��߷��ӣ�д����Ӧ�Ļ�ѧ����ʽ___��
��5��д��D��E��Ӧ�Ļ�ѧ����ʽ___��