��Ŀ����
����Ŀ����Ũ�ȵ��������һԪ���ᣬ������������ƽ�⣺HF![]() H++F-��HF+F-
H++F-��HF+F-![]() HF2- (���ȶ�)��25��ʱ����ͬ���������µ�2.0amol��L-1HF��Һ�У�c(HF)��c(F-)����ҺpH(��������仯)�ı仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��
HF2- (���ȶ�)��25��ʱ����ͬ���������µ�2.0amol��L-1HF��Һ�У�c(HF)��c(F-)����ҺpH(��������仯)�ı仯��ϵ��ͼ��ʾ������˵����ȷ���ǣ� ��
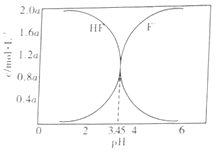
A.c(HF)+c(F-)=2.0amol��L-1
B.c(F-)>c(HF)ʱ����Һһ���ʼ���
C.������ҺpH����![]() ��������
��������
D.25��ʱ��HF�ĵ��볣��Ka=10-3.45
���𰸡�D
��������
A������Һ�к�F���У�F-��HF��![]() �����������غ��֪��
�����������غ��֪��![]() ����A����
����A����
B����ͼ��֪������ҺpH>3.45ʱ��c(F-)>c(HF)����˵�c(F-)>c(HF)ʱ����Һ��һ���ʼ��ԣ���B����
C��������ҺpH����c(F-)������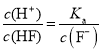 ���¶Ȳ��䣬Ka���䣬���
���¶Ȳ��䣬Ka���䣬���![]() ��С����C����
������C����
D����pH=3.45ʱ��c(F-)=c(HF)��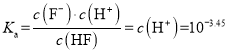 ����D��ȷ��
����D��ȷ��
�ʴ�Ϊ��D��

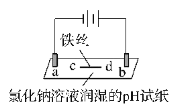
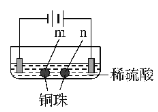
����Ŀ����ʯī�缫������е��ʵ�顣
ʵ��һ | ʵ��� | |
װ�� |
|
|
���� | a ��d����ֽ������b����죬�ֲ���ɫ��c�������Ա仯 | ����ʯī�缫�����������ݲ�����ͭ��n���Ҳ������ݲ����� |
���ж�ʵ������Ľ��ͻ��Ʋⲻ��������(�� ��)
A. ����ʵ��һ��ԭ����ʵ�����ͭ��m�����
B. a��d����2H2O��2e��=H2����2OH��
C. b����2Cl����2e��=Cl2����Cl2 + H2O =HCl + HClO
D. c�������˷�Ӧ��Fe��2e��=Fe2��
����Ŀ��I�������г��˼������ʣ��������ʵ������д�±���
���� | ͬλ�� | ͬ�������� | ͬ���칹�� | ͬϵ�� |
��� | __ | __ | __ | __ |
�� ![]() ��
��![]() ��
�� ![]() ��
��![]() �۽��ʯ��ʯī ��12C��13C��14C ��
�۽��ʯ��ʯī ��12C��13C��14C ��![]() ��
��![]()
II����ͼ��A��B��C �ֱ����������Ľṹģ�ͣ�

��ش��������⣺
(1)A �ĵ���ʽ______________��B �Ľṹ��ʽ________________��
(2)A����ͬϵ��ķ���ʽ����ͨʽ_____________(�� n ��ʾ)���� n��____________ʱ��������ʼ����ͬ���칹�壻�� n=6 ʱ��ͬ���칹����__________�֡�
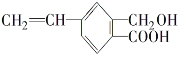
(3)A��B��C �����л����У�����ԭ�Ӿ��������___________(������)���ṹ��ʽΪ 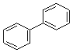 ���л����У�����ͬһƽ���ڵ�ԭ�������Ϊ__________������ ͬһƽ���ڵ�̼ԭ��������Ϊ____________��
���л����У�����ͬһƽ���ڵ�ԭ�������Ϊ__________������ ͬһƽ���ڵ�̼ԭ��������Ϊ____________��
(4)�л��� C �����еĽṹ��������_____________(����ĸ���)��
a����̼̼˫����̼̼��������Ľṹ b���ж���������ˮ���ܶȱ�ˮС
c������ʹ���� KMnO4 ��Һ����ˮ��Ӧ��ɫ d��һ������������������������Ӧ
(5)�������������л�����ȫȼ������ H2O �� CO2���������������(��ͬ״����)������_______(��A ��B �� C)��
III��ij�л���Ľṹ��ʽ��ͼ��1mol ���л��������Ժ�______mol������Ӧ�������Ժ�_____molNaOH ��Ӧ��