��Ŀ����
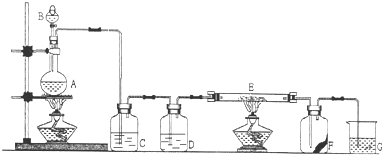
ͼ5��ʵ������ȡCl2����Cl2Ϊԭ�Ͻ����ض���Ӧ��ʵ�飺

ͼ5
��1��AΪ��������װ�ã�д����Ӧ�Ļ�ѧ����ʽ��_________________________________��
��2��ʵ�鿪ʼ�ȵ�ȼA���ľƾ��ƣ�������K����Cl2��������װ�ã��ٵ�ȼD���ľƾ��ƣ�����Eװ�á�Cl2ͨ��Cƿ�����D��Dװ����ʢ��̼�ۣ�����������ԭ��Ӧ������CO2��HCl��g������д��Dװ���з�Ӧ�Ļ�ѧ����ʽ_______________________��װ��C��������_________________________________��
��3��E��ʯ����Һ��������_____________________________________________________����ԭ����______________________________________________________________________��
��4������E����Һ��Ϊʯ��ˮ����Ӧ���̵�������_________________________________��
a.�а�ɫ��������
b.�ް�ɫ��������
c.�����ɰ�ɫ������Ȼ�������ʧ
��5��D����Ӧ��Ϻر�����K����ȥ�ƾ��ƣ��������ȵ����ã�A����������Cl2��������ʱB�е�������________________________��B��������
___________________________��
��1��MnO2+4HCl��Ũ��![]() MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
��2��2Cl2+C+2H2O![]() 4HCl+CO2 �ṩD�������ˮ����
4HCl+CO2 �ṩD�������ˮ����
��3��ʯ����Һ�ȱ��ɫ�������ɫ ���ɵ�HClʹʯ����Һ��죬δ��Ӧ���Cl2��H2O���ò���HClO��HClO��Ư������ʹ��ɫ��ʧ
��4��b
��5��Bƿ��Һ���½���ͬʱ����©����Һ������ ��������Cl2������Cl2�Ի�������Ⱦ
����:
ʵ��������ȡCl2����MnO2��Ũ�����ڼ��������·�Ӧ����Ӧԭ����MnO2+4HCl��Ũ��![]() MnCl2+Cl2��+2H2O
MnCl2+Cl2��+2H2O
��2����������CO2��HCl��g����������H2O��Cl2��Cͬʱ���뷴Ӧ���䷴Ӧ����ʽΪ��2Cl2+C+2H2O![]() 4HCl+CO2��װ��C���������ṩD�������ˮ������
4HCl+CO2��װ��C���������ṩD�������ˮ������
��3��ʯ����Һ�ȱ��ɫ�������ɫ�����ɵ�HClʹʯ����Һ��졣δ��Ӧ���Cl2��H2O���ò���HClO��HClO��Ư������ʹ��ɫ��ʧ��
��4���ӣ�2����Ӧ���ѷ�����CO2��HClͬʱ������ʯ��ˮ����HCl��CO2�������Ϊ4��1����Ȼ�����������������CaCO3�������ʴ�Ϊb��
��5������Cl2����Bƿ������ƿ��ѹǿ���� Bƿ��Һ���½���ͬʱ����©����Һ��������B����������������Cl2������Cl2�Ի�������Ⱦ��

 ��ͼ��ʵ������ȡ���ռ�Cl2��װ�ã�A��Cl2����װ�ã�E��Ӳ�ʲ�������װ��ϸ��˿����FΪ����Ĺ��ƿ���ձ�GΪβ������װ�ã�
��ͼ��ʵ������ȡ���ռ�Cl2��װ�ã�A��Cl2����װ�ã�E��Ӳ�ʲ�������װ��ϸ��˿����FΪ����Ĺ��ƿ���ձ�GΪβ������װ�ã�

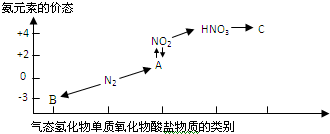 ��ͼ�ǵ�Ԫ�صļ��ּ�̬���������Ķ�Ӧ��ϵ��
��ͼ�ǵ�Ԫ�صļ��ּ�̬���������Ķ�Ӧ��ϵ��