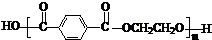��Ŀ����
��13�֣����ǻ�����ͬһ��̼ԭ�������Զ�ʧˮ����ȩ��ͪ
��1����������ԭ������д�� ��NaOH��ˮ��Һ���ȵĻ�ѧ����ʽΪ��
��
��NaOH��ˮ��Һ���ȵĻ�ѧ����ʽΪ��
��
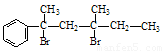 ��NaOH�Ĵ���Һ���ȿ������� ���ȶ��Ķ�ϩ����
��NaOH�Ĵ���Һ���ȿ������� ���ȶ��Ķ�ϩ����
��2��ȩͪ��ˮ�п�����ˮ��� �� ����һ�����淴Ӧ��ƽ��״̬��ƽ���λ�ã�������ȩͪ�Ľṹ����
�� ����һ�����淴Ӧ��ƽ��״̬��ƽ���λ�ã�������ȩͪ�Ľṹ����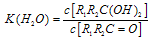 Ϊȩͪ��ˮ��ƽ�ⳣ������ֵԽ�����Ӧ��ȩͪˮ����Խ�ȶ����±��Dz���ȩ��ͪ��ˮ��ƽ�ⳣ����
Ϊȩͪ��ˮ��ƽ�ⳣ������ֵԽ�����Ӧ��ȩͪˮ����Խ�ȶ����±��Dz���ȩ��ͪ��ˮ��ƽ�ⳣ����
|
������ |
|
|
|
|
|
K(H2O) |
2��103 |
1.3 |
0.71 |
8.3��10-3 |
|
������ |
|
|
|
|
|
K(H2O) |
2��10-3 |
2.9 |
10 |
�ܴ� |
�������ϱ������ݷ��������ܽ���������ۣ�
�� ��
�� ��
��3����ҵ����������ȩ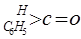 ���������ַ�����
���������ַ�����

�뷽������ȣ������ڵ��ŵ��� ��
ȱ���� ��
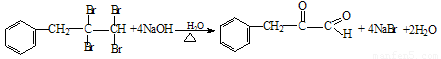
��4���������л�Ⱦ�ϵ���Ҫԭ�ϣ���ȩ����ͪ���뱽�����ɱ���ķ�ӦΪ��

��Ӧ�ٵ�ԭ��������Ϊ100%�����м����A�Ľṹ��ʽΪ ��
��Ӧ�ڵķ�Ӧ�������� ��
��5���ɶԱ�����ȩ����������Ա������ᡱ�롰�Ҷ��������ۣ����ɵľ�����ά�����ڣ�
�Ľṹ��ʽΪ ��
��13�֣�
��1����дΪ��ˮ��Ӧ��NaOHд�ڼ�ͷ�ϡ�����ΪHBrҲ���ԣ�����2�֣� 5�֣�1�֣�
��2�����£����������������������֣���2�֣�ÿ��1�֣�
a.�ʻ�̼�����ӵļ�Խ�࣬���Ӧ��ȩͪˮ����Խ���ȶ���
b.�ʻ�̼����������ʱ�����������Խ��ˮ����Խ���ȶ���
c. �ʻ�̼���ڵ�̼��������±ԭ��ʹˮ�����ø����ȶ���
d. �ʻ�̼���ڵ�̼��������±ԭ��Խ�ࡢ±ԭ�ӷǽ�����Խǿ����ˮ����Խ�ȶ������ʻ�������̼ԭ������±�غ�̼ԭ�����µ���ԭ��Խ�࣬��ˮ����Խ���ȶ���
��3��������δʹ�������ж�ԭ�ϣ����������Ʒ����һ����������Ⱦ��ˮ��1�֣� �����ڱȷ�������Ҫ���¶ȸߣ���Ժ��ܽ϶ࣨ1�֣�
��4��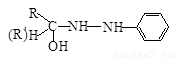 ��2�֣� ��ȥ��Ӧ��1�֣�
��2�֣� ��ȥ��Ӧ��1�֣�
��5��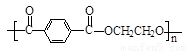 ��
��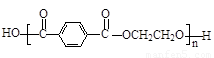 ��2�֣�
��2�֣�
��������

��13�֣����ǻ�����ͬһ��̼ԭ�������Զ�ʧˮ����ȩ��ͪ
��1����������ԭ������д����NaOH��ˮ��Һ���ȵĻ�ѧ����ʽΪ�� ��
��NaOH�Ĵ���Һ���ȿ������� ���ȶ��Ķ�ϩ����
��2��ȩͪ��ˮ�п�����ˮ����� ����һ�����淴Ӧ��ƽ��״̬��ƽ���λ�ã�������ȩͪ�Ľṹ����
Ϊȩͪ��ˮ��ƽ�ⳣ������ֵԽ�����Ӧ��ȩͪˮ����Խ�ȶ����±��Dz���ȩ��ͪ��ˮ��ƽ�ⳣ����
| ������ |
|
|
|
|
| K(H2O) | 2��103 | 1.3 | 0.71 | 8.3��10-3 |
| ������ |
|
|
|
|
| K(H2O) | 2��10-3 | 2.9 | 10 | �ܴ� |
�������ϱ������ݷ��������ܽ���������ۣ�
�� ��
�� ��
��3����ҵ����������ȩ![]() ���������ַ�����
���������ַ�����
�뷽������ȣ������ڵ��ŵ��� ��
ȱ���� ��
��4���������л�Ⱦ�ϵ���Ҫԭ�ϣ���ȩ����ͪ���뱽�����ɱ���ķ�ӦΪ��
��Ӧ�ٵ�ԭ��������Ϊ100%�����м����A�Ľṹ��ʽΪ ��
��Ӧ�ڵķ�Ӧ�������� ��
��5���ɶԱ�����ȩ����������Ա������ᡱ�롰�Ҷ��������ۣ����ɵľ�����ά�����ڣ�
�Ľṹ��ʽΪ ��
��13�֣����ǻ�����ͬһ��̼ԭ�������Զ�ʧˮ����ȩ��ͪ
��1����������ԭ������д��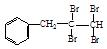 ��NaOH��ˮ��Һ���ȵĻ�ѧ����ʽΪ�� ��
��NaOH��ˮ��Һ���ȵĻ�ѧ����ʽΪ�� ��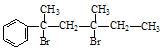 ��NaOH�Ĵ���Һ���ȿ������� ���ȶ��Ķ�ϩ����
��NaOH�Ĵ���Һ���ȿ������� ���ȶ��Ķ�ϩ����
��2��ȩͪ��ˮ�п�����ˮ���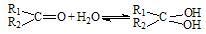 ������һ�����淴Ӧ��ƽ��״̬��ƽ���λ�ã�������ȩͪ�Ľṹ����
������һ�����淴Ӧ��ƽ��״̬��ƽ���λ�ã�������ȩͪ�Ľṹ����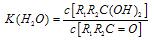 Ϊȩͪ��ˮ��ƽ�ⳣ������ֵԽ�����Ӧ��ȩͪˮ����Խ�ȶ����±��Dz���ȩ��ͪ��ˮ��ƽ�ⳣ����
Ϊȩͪ��ˮ��ƽ�ⳣ������ֵԽ�����Ӧ��ȩͪˮ����Խ�ȶ����±��Dz���ȩ��ͪ��ˮ��ƽ�ⳣ����
| ������ |  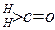 | 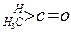 | 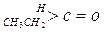 | 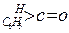 |
| K(H2O) | 2��103 | 1.3 | 0.71 | 8.3��10-3 |
| ������ | 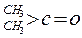 | 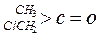 | 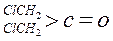 | 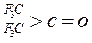 |
| K(H2O) | 2��10-3 | 2.9 | 10 | �ܴ� |
�� ��
�� ��
��3����ҵ����������ȩ
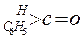 ���������ַ�����
���������ַ�����
�뷽������ȣ������ڵ��ŵ��� ��
ȱ���� ��
��4���������л�Ⱦ�ϵ���Ҫԭ�ϣ���ȩ����ͪ���뱽�����ɱ���ķ�ӦΪ��

��Ӧ�ٵ�ԭ��������Ϊ100%�����м����A�Ľṹ��ʽΪ ��
��Ӧ�ڵķ�Ӧ�������� ��
��5���ɶԱ�����ȩ����������Ա������ᡱ�롰�Ҷ��������ۣ����ɵľ�����ά�����ڣ�
�Ľṹ��ʽΪ ��


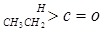
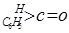
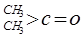
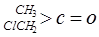
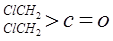
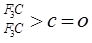
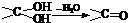 ��
��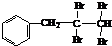 ��NaOH��ˮ��Һ���ȵĻ�ѧ����ʽΪ��
��NaOH��ˮ��Һ���ȵĻ�ѧ����ʽΪ��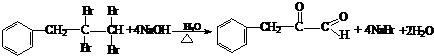
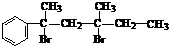 ��NaOH���Ҵ���Һ���ȣ���������
��NaOH���Ҵ���Һ���ȣ��������� ������һ�����淴Ӧ��ƽ��״̬��ƽ���λ�ã�������ȩͪ�Ľṹ����
������һ�����淴Ӧ��ƽ��״̬��ƽ���λ�ã�������ȩͪ�Ľṹ����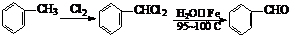 ����
����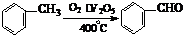
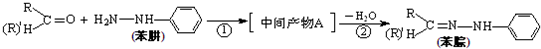
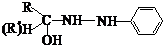
 ��
��