��Ŀ����
���û�����������ɰ�ķ���--��þ�����ȡ��ˮ����þ��MgSO4?7H2O������þ�����Ҫ�ɷ���MgCO3���������������ʣ�MgO��SiO2��Fe2O3��FeO��CaO��Al2O3��MnO�ȣ���
��1������������������������ʽ��ȫ����ʱ��Һ��pH
| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Mn��OH��2 | Mg��OH��2 |
| pHֵ | 5.2 | 3.2 | 9.7 | 10.4 | 11.2 |
| �¶�/�� | 10 | 30 | 40 | 50 | 60 |
| CaSO4 | 0.19 | 0.21 | 0.21 | 0.20 | 0.19 |
| MgSO4?7H2O | 30.9 | 35.5 | 40.8 | 45.6 | / |
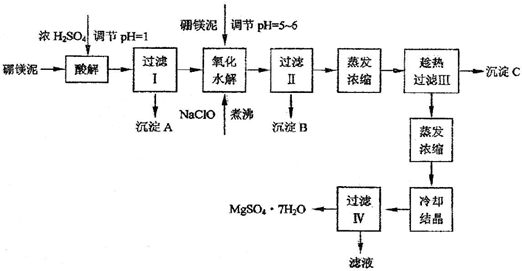
������������ͼ���ο�pH���ݺ��ܽ�����ݣ��Իش��������⣺
��1����þ�����Ũ����ʱ��FeO�����ı仯��______��ѡ����ţ���
a�����ۻ�����������b����������������c�����ܽ�
��2�������ˢ�õ�����Һ�м�����þ�࣬������Һ��pH=5��6���ټ���NaClO��Һ�ɽ���Һ�е�Mn2+������MnO2���÷�Ӧ�����ӷ�Ӧ����ʽΪ______��������е�Ŀ����_______��
��3������B�г�MnO2�����______���ѧʽ�������ʣ�
��4������C�Ļ�ѧʽ��______����������C�IJ�������Ƚ��е�ԭ����______��ϴ�ӳ�����Ҫ�IJ��������У��ձ���______�������Ҵ�����ˮ��ϴ�Ӽ�ϴ�ӳ���C��ԭ����______��
��5�����˳�MgSO4?7H2O�������Һ�к��еĽ�����������______�������жԸ���Һ�Ĵ���������______��������Һ�Ƿ���SO42-������______��
��2����������֪�����������ӷ�Ӧ�����ɶ������̣���������������������ӣ����ԭ���غ�ã������ӷ�Ӧ����ʽΪ��Mn2++ClO-+H2O�TMnO2��+2H++Cl-�������Ӻ�������ˮ�������ȷ�Ӧ�������ܴٽ������Ӻ�������ˮ�⣬�Ӷ��������������������������ʴ�Ϊ��Mn2++ClO-+H2O�TMnO2��+2H++Cl-���ٽ�Al3+��Fe3+ˮ�⣻
��3�����ݱ�1֪��pH=5-6ʱ����������������������ȫ���ɳ���������B�����ijɷֻ���Fe��OH��3��Al��OH��3
���ʴ�Ϊ��Fe��OH��3��Al��OH��3��
��4�����ݱ�2֪������������¶ȵ��������ܽ�Ƚ��ͣ����Գ���C������ƣ�
�¶�Խ������þ���ܽ��ԽС��Ϊ��������þ������������Ƶ��ܽ⣬��������C�IJ�������Ƚ��У�
ϴ�ӳ�����Ҫ�IJ��������У���������õ�©�����������������õIJ�������
����������ܼ��е��ܽ�ȴ������л��ܼ��е��ܽ�ȣ�����Ϊ��������Ƶ��ܽ�ȣ����Ҵ�����ˮ��ϴ�Ӽ�ϴ�ӳ���C��
�ʴ�Ϊ��CaSO4?2H2O��CaSO4���Է�MgSO4���¶ȵ�ʱ�ᾧ������©����������������CaSO4?2H2O���ܽ�ȣ������CaSO4?2H2O���ܽ⣩��
��5������Ĵ��������к��������ӣ������ƺ�����þ�ǿ������Σ����Թ��˳�MgSO4?7H2O�������Һ�к��еĽ�����������Mg2+��Na+��
��Һ�к���þ���ӣ�������ԭ������Ҫþ���ӣ�����Ϊ������Դ���˷ѣ�þ���ӿ���ѭ�����ã�
��������ӵļ��鷽���ǣ�ȡ��Һ1��2 mL���Թ��У�����BaCl2��Һ�����а�ɫ�������ɣ�˵����SO42-������û�У�
�ʴ�Ϊ��Mg2+��Na+��ѭ�����ã�ȡ��Һ1��2 mL���Թ��У�����BaCl2��Һ�����а�ɫ�������ɣ�˵����SO42-��
��������1�����������ǽ�����������������л�ԭ�ԣ����ݽ���������ͻ�ԭ�����ӵ�����ȷ���������������ı仯��
��2������������ǿ�����ԣ����������ӱ��������ɶ������̣�ͬʱ��������ԭ����������ˮ�������ȷ�Ӧ���¶�Խ�ߣ�ˮ��̶�Խ��
��3�����ݱ�1ȷ������B�ijɷ֣�
��4�����ݱ�2ȷ������C�ijɷ֣���������þ���¶ȵĹ�ϵ��������������������ѡȡ������������������ԭ��֪���������ˮ�е��ܽ�ȴ������Ҵ��е��ܽ�ȣ�
��5����Һ�д��ڿ����Ե������ӣ�Ϊ��Լ��Դ��Ӧѭ�����ã��������ữ���Ȼ���������������ӣ�
���������⿼�黯ѧ�뼼�����漰���ӵļ��鷽����������ѡȡ�����ʵķ����֪ʶ�㣬ע���Ķ������Ϣ�����������Ϣ���н���ѶȽϴ�

 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�A�������ʽṹ�����ʡ�
��1��ǰ������Ԫ���е�һ��������С���� _______ (��Ԫ�ط���)�����̬ԭ�ӵĵ����Ų�ʽΪ _______ ���ڶ����ڷǽ���Ԫ���γɵ��⻯���л�ѧ������������ _______
(�����ʽ)����������CCl4�е��ܽ�ȱ���ˮ�е��ܽ�� _______ (���С��)��
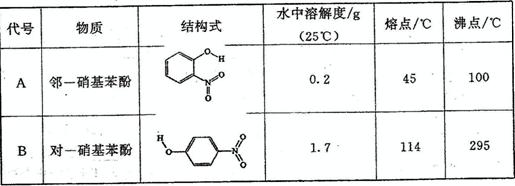 |
��2�������γɷ��Ӽ�����ͷ�����������������ʵ�Ӱ�����������졣�����±����ݣ��γɷ��Ӽ������������ _______ (��������ĸ����)��
��3�������ܵĴ�С��MgO _______ NaCl�����ܵĴ�С��HBr _______ HI��(�>������������<��)
��4���������ʵ��۵�ߵ�˳����ȷ���� _______
A�����ʯ>�����>��������>̼����
B��CI4 > CBr4 > CCl4 > CH4
C��SiF4 > NaF > NaCl > NaBr
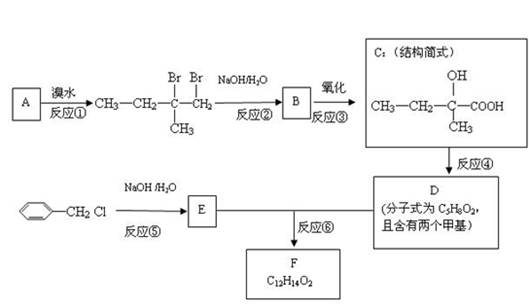
B��ʵ�黯ѧ��
��ˮ����þ(MgSO4��7H2O)��ӡȾ����ֽ��ҽҩ�ȹ�ҵ�϶��й㷺��Ӧ�ã����û�����������ɰ�ķ�����һ��þ�����ȡ��ˮ����þ����þ�����Ҫ�ɷ���MgCO3����������������(MgO��SiO2��Fe2O3��FeO��CaO��Al2O3��MnO�ȣ���
��1 ����������������������ʽ��ȫ����ʱ��Һ��pH
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 | Mg(OH)2 |
| pHֵ | 5.2 | 3.2 | 9.7 | 10.4 | 11.2 |
��2 �����ε��ܽ��(��λΪg��100gˮ)
| �¶� / �� | 10 | 30 | 40 | 50 | 60 |
| CaSO4 | 0.19 | 0.21 | 0.21 | 0.20 | 0.19 |
| MgSO4��7H2O | 30.9 | 35.5 | 40.8 | 45.6 | / |
��þ����ȡ��ˮ����þ�Ĺ����������£�

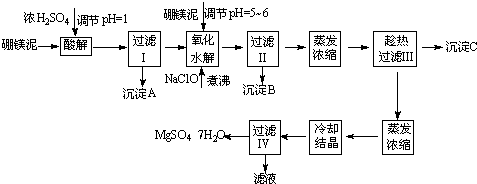
������������ͼ���ο�����pH���ݺ��ܽ�����ݣ��Իش��������⣺
��1������I����Һ�м�����þ�࣬������Һ��pH��5��6���ټ���NaClO��Һ������У�����Һ�е�Mn2+������MnO2����Ӧ�����ӷ�Ӧ����ʽΪ _______ ��������е���ҪĿ����_ _______ ��
��2������B�г�MnO2��SiO2����� _______ (�ѧʽ)�����ʡ�
��3��������ˢ�����Һ���Ƿ���Fe3����ʵ�鷽���� _______ ��
��4������C�Ļ�ѧʽ�� _______ ������II����ȹ��˵������� _______
��ˮ����þ(MgSO4��7H2O)��ӡȾ����ֽ��ҽҩ�ȹ�ҵ�϶��й㷺��Ӧ�ã����û�����������ɰ�ķ�������þ�����ȡ��ˮ����þ����þ�����Ҫ�ɷ���MgCO3����������������(MgO��SiO2��Fe2O3��FeO��CaO��Al2O3��MnO�ȣ���
��1 ����������������������ʽ��ȫ����ʱ��Һ��pH
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 | Mg(OH)2 |
| pHֵ | 5.2 | 3.2 | 9.7 | 10.4 | 11.2 |
| �¶� / �� | 10 | 30 | 40 | 50 | 60 |
| CaSO4 | 0.19 | 0.21 | 0.21 | 0.20 | 0.19 |
| MgSO4��7H2O | 30.9 | 35.5 | 40.8 | 45.6 | / |
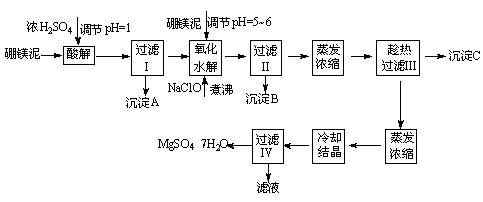
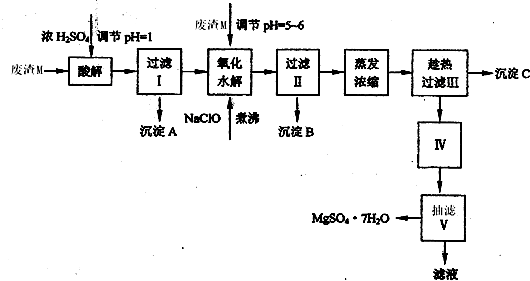
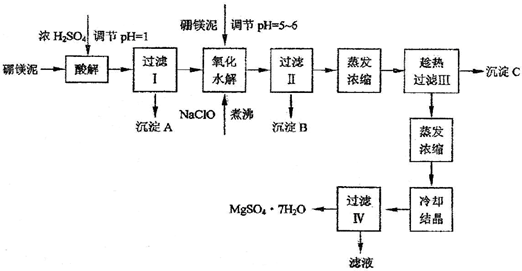
������������ͼ���ο�����pH���ݺ��ܽ�����ݣ��Իش��������⣺
��1������I����Һ�м�����þ�࣬������Һ��pH��5��6���ټ���NaClO��Һ������У�����Һ�е�Mn2+������MnO2����Ӧ�����ӷ�Ӧ����ʽΪ ��������е���ҪĿ����_ �� ��2������B�г�MnO2��SiO2����� (�ѧʽ)�����ʡ�
��3��������ˢ�����Һ���Ƿ���Fe3����ʵ�鷽���� ��
��4������C�Ļ�ѧʽ�� ������III����ȹ��˵������� ��