��Ŀ����
��п�������Ĺ�ҵ��������п�������˺�Zn�⣬������Fe��Al��Cd��SiO2�����ʣ�������п����ȡ������ZnSO4��7H2O�ͽ����ӣ�Cd����һ������ij��ԣ����������£�
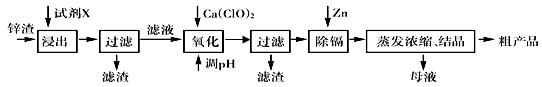
��֪��Fe3+��Al3+��Zn2+��Cd2+��Fe2+������������ȫ����ʱ��pH�ֱ�Ϊ��3.2��4.7��6.5��9.4��9.7���Իش��������⣺
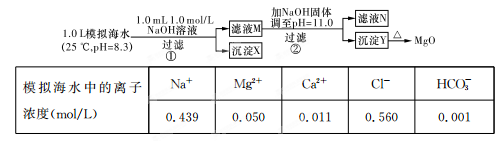
��1������ʱ�õ����Լ�XΪ �� д���ܽ�����мӿ�������ʺ���߽����ʵ������ʩ��_______________________________��______________________________��
��2��д������Ca(ClO)2��Ӧ�����ӷ���ʽ ��
��3������pH���̿���ѡ�� ���ZnO����NaOH�����������̵�pH����һ�����5����Ŀ���� ��
��4��д�����˺���������п�۷�Ӧ�����ӷ���ʽ ��
��5��������Ũ������ʱ��Ҫ��ȡʵ���ʩ�ǣ� ��

��֪��Fe3+��Al3+��Zn2+��Cd2+��Fe2+������������ȫ����ʱ��pH�ֱ�Ϊ��3.2��4.7��6.5��9.4��9.7���Իش��������⣺
��1������ʱ�õ����Լ�XΪ �� д���ܽ�����мӿ�������ʺ���߽����ʵ������ʩ��_______________________________��______________________________��
��2��д������Ca(ClO)2��Ӧ�����ӷ���ʽ ��
��3������pH���̿���ѡ�� ���ZnO����NaOH�����������̵�pH����һ�����5����Ŀ���� ��
��4��д�����˺���������п�۷�Ӧ�����ӷ���ʽ ��
��5��������Ũ������ʱ��Ҫ��ȡʵ���ʩ�ǣ� ��
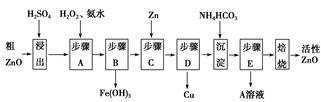
��1��H2SO4��Һ �ʵ����¡���ֽ��衢�ӳ��ܽ�ʱ��ȣ������𰸺���Ҳ���֣�
��2��2H++ClO��+2Fe2+ =Cl��+2Fe3++H2O����ƽ����1�֣�
��3��ZnO ����ȥFe3+��Al3+����ֹZn2+��������
��4��Zn+Cd2+=Zn2++Cd ��5������һ������ȣ������������Ҳ���֣�
��2��2H++ClO��+2Fe2+ =Cl��+2Fe3++H2O����ƽ����1�֣�
��3��ZnO ����ȥFe3+��Al3+����ֹZn2+��������
��4��Zn+Cd2+=Zn2++Cd ��5������һ������ȣ������������Ҳ���֣�
�����������1���������Ʊ�����п����ĵĹ������̣�п���к������ܵĶ����������ʣ�ѡ�����ܳ�ȥ�������裬���Ǿ������������ʣ�Ӧѡ������������ӵ�������Һ�����ܽ⡣��Ҫ�ӿ�������ʺ���߽����ʣ������ʵ������¶Ȼ������Һ���ʵ��ӳ�ʱ��ȡ�
��2���������������Һ�к�������Fe3+��Al3+��Zn2+��Cd2+��Fe2+ �����ӣ�������Щ���ӷֱ������pHֵ��Ӧ�ð�Fe2+ ����ΪFe3+ �ڳ�����ȥ�����Լ���������Ca(ClO)2 �����ķ�Ӧ��ʽΪ��2H++ClO��+2Fe2+ =Cl��+2Fe3++H2O��
��3���������������Ʊ���������п���壬�����ڵ���pHʱҪע�ⲻ���������ʣ�����Ӧ��ѡ��ZnO���ڣ�������pHֵ��5����Ŀ����ʹ���׳�����Fe3+��Al3+ �ȳ�����ȥ����ֹpH���߰����ɵ�Al(OH)3�ܽ⡢��Zn2+ ������
��4�����ϲ�������pH����Һ�л�������������Cd2+ ������Cd2+ ������pHֵ��Zn2+ ������pHֵ��������Բ����ó�������ȥ������Ҫ���뵥��Zn��Cd�û���������˷��������ӷ���ʽΪ��Zn+Cd2+=Zn2++Cd��
��5������п������Ũ��ʱ������ˮ�⣬����Ϊ�˼���ˮ��������ʣ�Ӧ�ñ�����Һһ������ȡ�

��ϰ��ϵ�д�
�����Ŀ
 Sr2��(aq)��SO42��(aq)��Ksp��2.5��10��7
Sr2��(aq)��SO42��(aq)��Ksp��2.5��10��7
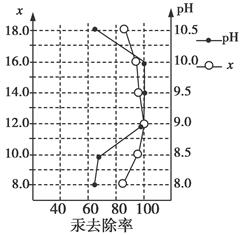
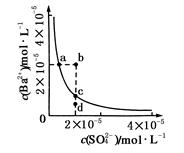
 Ba2��(aq)��SO42-(aq)��Ksp��c(Ba2��)��c(SO42-)�������ܽ�ƽ��������ͼ��ʾ������˵����ȷ����
Ba2��(aq)��SO42-(aq)��Ksp��c(Ba2��)��c(SO42-)�������ܽ�ƽ��������ͼ��ʾ������˵����ȷ����