��Ŀ����
����14�֣�ijͬѧ������װ��(�̶�����������������)�����йذ�����ȡ�������ʵ���̽�����ش��������⣮
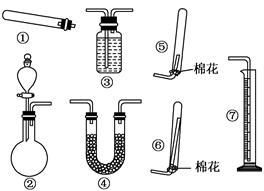
��1������װ�â���ȡNH3���䷴Ӧ�Ļ�ѧ����ʽΪ ����Ҫ�ⶨ���ɵ�NH3������������ѡ���װ���� (��װ�����)��װ������ʢ�Լ�Ӧ���е������� ��
��2������װ�â���ȡ���ռ������NH3���ռ�װ��Ӧѡ�� (��װ�����)��
��3����ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ�����ֱ����1��10nm֮�䣩��
����ŨCaCl2��Һ��ͨ��NH3��CO2����������̼���ʱ��Ӧ��ͨ��������� ����д��������̼��Ƶ����ӷ���ʽ ��
����ŨCaCl2��Һ��NH3������ȷ������£�CO2�����������ᵼ������̼��Ʋ����½�����CO2������Һ�д������ڵ������У�������������ʵĵ��������ˮ��������������ӣ�_____________________________��

��1������װ�â���ȡNH3���䷴Ӧ�Ļ�ѧ����ʽΪ ����Ҫ�ⶨ���ɵ�NH3������������ѡ���װ���� (��װ�����)��װ������ʢ�Լ�Ӧ���е������� ��
��2������װ�â���ȡ���ռ������NH3���ռ�װ��Ӧѡ�� (��װ�����)��
��3����ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ�����ֱ����1��10nm֮�䣩��
����ŨCaCl2��Һ��ͨ��NH3��CO2����������̼���ʱ��Ӧ��ͨ��������� ����д��������̼��Ƶ����ӷ���ʽ ��
����ŨCaCl2��Һ��NH3������ȷ������£�CO2�����������ᵼ������̼��Ʋ����½�����CO2������Һ�д������ڵ������У�������������ʵĵ��������ˮ��������������ӣ�_____________________________��
��ÿ��2�֣���14�֣�
��1��2NH4Cl��Ca(OH)2 2NH3����CaCl2��2H2O���ۢ�
2NH3����CaCl2��2H2O���ۢ�
���������ڸ��Լ������Լ����ӷ������백����Ӧ��
��2����
��3����NH3�� Ca2++2NH3+H2O+CO2=CaCO3+2NH4+
��Ca2+ HCO3- NH Cl-
Cl-
��1��2NH4Cl��Ca(OH)2
 2NH3����CaCl2��2H2O���ۢ�
2NH3����CaCl2��2H2O���ۢ����������ڸ��Լ������Լ����ӷ������백����Ӧ��
��2����
��3����NH3�� Ca2++2NH3+H2O+CO2=CaCO3+2NH4+
��Ca2+ HCO3- NH
 Cl-
Cl- ��1��ʵ������ȡ�����ķ���ʽ��2NH4Cl��Ca(OH)2 2NH3����CaCl2��2H2O��Ҫ�ⶨ���ɵİ������������Ҫͨ����Һ�������װ������ʢ�ŵ�Һ��������㰱�������ڸ��Լ������Լ����ӷ����Ҳ��백����Ӧ��
2NH3����CaCl2��2H2O��Ҫ�ⶨ���ɵİ������������Ҫͨ����Һ�������װ������ʢ�ŵ�Һ��������㰱�������ڸ��Լ������Լ����ӷ����Ҳ��백����Ӧ��
��2��������������ˮ�����������ܶ�С�ڿ����ģ����Բ��������ſ������ռ�����ѡ��װ�âޡ�
��3��������CO2������ˮ����������������ˮ����������ͨ����ǰ������Ʒ�Ӧ�����ӷ���ʽ��Ca2++2NH3+H2O+CO2=CaCO3+2NH4+��
�����CO2������������̼����ƣ�������Һ�д������ڵ�������Ca2+��HCO3-��NH ��Cl-��
��Cl-��
 2NH3����CaCl2��2H2O��Ҫ�ⶨ���ɵİ������������Ҫͨ����Һ�������װ������ʢ�ŵ�Һ��������㰱�������ڸ��Լ������Լ����ӷ����Ҳ��백����Ӧ��
2NH3����CaCl2��2H2O��Ҫ�ⶨ���ɵİ������������Ҫͨ����Һ�������װ������ʢ�ŵ�Һ��������㰱�������ڸ��Լ������Լ����ӷ����Ҳ��백����Ӧ����2��������������ˮ�����������ܶ�С�ڿ����ģ����Բ��������ſ������ռ�����ѡ��װ�âޡ�
��3��������CO2������ˮ����������������ˮ����������ͨ����ǰ������Ʒ�Ӧ�����ӷ���ʽ��Ca2++2NH3+H2O+CO2=CaCO3+2NH4+��
�����CO2������������̼����ƣ�������Һ�д������ڵ�������Ca2+��HCO3-��NH
 ��Cl-��
��Cl-��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
