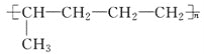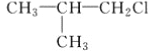��Ŀ����
����Ŀ�����ǵ��͵İ뵼����ϣ��ڹ������µ����Կ���߽�ǧ������ͼ�Ǵ�ij�������������뵼�����(��Ag2SeO��Cu ����)����ȡ�������Ĺ�������ͼ��
�ش��������⣺
(1)д�� Se ��ԭ�ӽṹʾ��ͼ_______��
(2)��֪��Ӧ������һ�ֿɲ������ѭ�������嵥�ʣ�д���÷�Ӧ�����ӳ�ʽ_______��
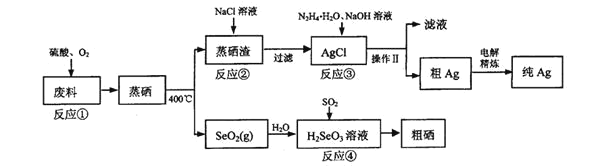
(3)��֪��������Ag2SO4��AgCl�ı�����Һ�������Ӻ�������Ũ�ȹ�ϵ��ͼ��ʾ����Ӧ��Ϊ_______(д���ӷ���ʽ)���÷�Ӧ�Ļ�ѧƽ�ⳣ����������Ϊ_______��

(4)д����Ӧ�ܵĻ�ѧ����ʽ________��
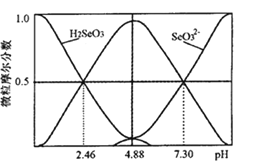
(5)�����£�H2SeO3ˮ��Һ�е�H2SeO3��HSeO3-��SeO32-Ħ�������� pH �ı仯��ͼ ��ʾ���������� SeO32-�� Kh_______��
(6)��ҵ�ϴ�����⾫��ʱ�����Һ��pHΪ1.5��2������ǿ��Ϊ5��10A�������ҺpH̫С����⾫�����������������������ӷŵ磬���ᷢ��_______(д�缫��Ӧʽ)������ 10A �ĵ������ 60min �õ�32.4gAg����õ��صĵ��Ч��Ϊ_______(����С�����һλ)��ͨ��һ������ʱ������ʵ�ʳ����Ľ���������ͨ����ͬ����ʱ������Ӧ�����Ľ�������֮�Ƚе��Ч�ʣ������ڳ���Ϊ96500C��mol-1��
���𰸡�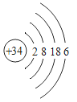 4AgCl+N2H4H2O+4OH-=4Ag+4Cl-+N2��+5H2O Ag2SO4(s)+2Cl-(aq)2AgCl(s)+SO42-(aq) 1014 H2SeO3+2SO2+H2O=2H2SO4+Se 10-6.7 2H++2e-=H2�� 80.4
4AgCl+N2H4H2O+4OH-=4Ag+4Cl-+N2��+5H2O Ag2SO4(s)+2Cl-(aq)2AgCl(s)+SO42-(aq) 1014 H2SeO3+2SO2+H2O=2H2SO4+Se 10-6.7 2H++2e-=H2�� 80.4
��������
�������뵼�����(��Ag2Se��Cu����)���������ᡢͨ������������������ͭ����������SeO2���壬SeO2��ˮ��Ӧ����H2SeO3��ͨ�����������������ԭ��Ӧ����Se�������������Ȼ�����Һ����AgCl�����˼���N2H4H2O������������Һ������������ԭ��Ӧ����Ag����⾫�����ɵõ��������Դ˽����⡣
(1)SeΪ34��Ԫ�أ�λ�ڵ������ڵڢ�A�壬��ԭ�ӽṹʾ��ͼΪ�� ��
��
(2)��Ӧ������һ�ֿɲ������ѭ�������嵥�ʣ�Ӧ���ɵ������÷�Ӧ�������ӽ���Ԫ����������ϵ����غ��Ԫ���غ��֪��Ӧ�����ӷ���ʽΪ4AgCl+N2H4H2O+4OH-��4Ag+4Cl-+N2��+5H2O��
(3)��ͼ��֪������Ũ����ͬʱ���Ȼ���������Һ��������Ũ�ȸ��ͣ�˵���Ȼ��������׳��������Է�Ӧ��Ϊ����������ת��Ϊ�Ȼ����Ĺ��̣����ӷ���ʽΪ��Ag2SO4(s)+2Cl-(aq)2AgCl(s)+SO42-(aq)����ͼ��֪Ksp(Ag2SO4)=10-5��Ksp(AgCl)=10-9.75��Ag2SO4(s)+2Cl-(aq)��2AgCl(s)+SO42-(aq)�Ļ�ѧƽ�ⳣ��K=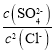 =
= = 1014.5����ѧƽ�ⳣ����������Ϊ1014��
= 1014.5����ѧƽ�ⳣ����������Ϊ1014��
(4)�������̿�֪��Ӧ��ΪH2SeO3��SO2���ù����ж������������������ᣬ��ϵ����غ��Ԫ���غ��֪����ʽΪH2SeO3+2SO2+H2O��2H2SO4+Se��
(5)������ SeO32-�� Kh=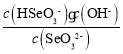 ����ͼ��֪pH=7.3ʱ����Һ��c(HSeO3-)=c(SeO32-)����ʱ��Һ��c(OH-)=10-6.7mol/L������Kh=10-6.7��
����ͼ��֪pH=7.3ʱ����Һ��c(HSeO3-)=c(SeO32-)����ʱ��Һ��c(OH-)=10-6.7mol/L������Kh=10-6.7��
(6)�����ҺpH̫С����������Ũ�Ƚϴ�⾫�����������������������ӷŵ磬���ᷢ��2H++2e-��H2������10A�ĵ������60min������ӵ����ʵ���Ϊ![]() mol=0.373mol�����ۿɵõ�0.373molAg�����õ�32.4gAg�����ʵ���Ϊ
mol=0.373mol�����ۿɵõ�0.373molAg�����õ�32.4gAg�����ʵ���Ϊ![]() =0.3mol����õ��صĵ��Ч��Ϊ
=0.3mol����õ��صĵ��Ч��Ϊ![]() ��100%=80.4%��
��100%=80.4%��