��Ŀ����
�����£�0.1m01��L��������Һ��
��HCl����CH3COOH����CH3COONa����NaOH����FeCl3����NaCl��
��1��pH��С��������˳��Ϊ ������ţ���
��2��ʵ�������Ƣݵ���Һʱ��������������ᣬ����õ����ǻ��ǵ���Һ���������ǵ�ԭ���ǣ������ӷ���ʽ��ʾ��
��3�����ʵ���Ũ����ͬ�Ģٰ�ˮ���Ȼ�梨�̼����梨�������梨������
������������Һ�У�笠��������ʵ���Ũ���ɴ�С��˳����
��4����֪��1molH��H����1molN��H����1molN��N���ֱ���Ҫ��������436kJ��39l kJ��946 kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ
��HCl����CH3COOH����CH3COONa����NaOH����FeCl3����NaCl��
��1��pH��С��������˳��Ϊ ������ţ���
��2��ʵ�������Ƣݵ���Һʱ��������������ᣬ����õ����ǻ��ǵ���Һ���������ǵ�ԭ���ǣ������ӷ���ʽ��ʾ��
��3�����ʵ���Ũ����ͬ�Ģٰ�ˮ���Ȼ�梨�̼����梨�������梨������
������������Һ�У�笠��������ʵ���Ũ���ɴ�С��˳����
��4����֪��1molH��H����1molN��H����1molN��N���ֱ���Ҫ��������436kJ��39l kJ��946 kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ
��1���٢ڢݢޢۢ� ��2��Fe3++3H2O Fe(OH)3+3H+
Fe(OH)3+3H+
��3���ݣ��ܣ��ڣ��ۣ��� ��4��N2(g)+3H2(g) 2NH3(g)
2NH3(g) 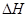 =-92.0kJ/mol
=-92.0kJ/mol
 Fe(OH)3+3H+
Fe(OH)3+3H+��3���ݣ��ܣ��ڣ��ۣ��� ��4��N2(g)+3H2(g)
 2NH3(g)
2NH3(g) 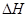 =-92.0kJ/mol
=-92.0kJ/mol�����������1��������һԪǿ�ᣬ������һԪ���ᣬ������������ǿ���Σ�����������һԪǿ��Ȼ�����ǿ�������Σ��Ȼ�����ǿ��ǿ���Σ�������Ũ����ȵ������£�pH��С��������˳��Ϊ�٢ڢݢޢۢܡ�
��2���Ȼ�������ˮ��������ˮ�������������������ᣬ���Լ��������Ŀ�������������ӵ�ˮ�⣬����ʽ��Fe3++3H2O
 Fe(OH)3+3H+��
Fe(OH)3+3H+����3������Һ��NH4��ˮ�⣬����������ܵ���������ӣ�����NH4��ˮ�⡣HCO3��ˮ�⣬�Լ��ԣ��ٽ�NH4��ˮ�⣬����������Һ�У�笠��������ʵ���Ũ���ɴ�С��˳���Ǣݣ��ܣ��ڣ��ۣ��١�
��4����Ӧ���Ƕϼ����յ��������γɻ�ѧ�����ų��������IJ�ֵ����÷�Ӧ�ķ�Ӧ�ȡ�H��3��436kJ/mol��946kJ/mol��2��3��391kJ/mol����92.0kJ/mol�����Է�Ӧ���Ȼ�ѧ����ʽ��N2(g)+3H2(g)
 2NH3(g)
2NH3(g) 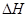 =-92.0kJ/mol��
=-92.0kJ/mol�������������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ����ֵ��ѧ��������û���֪ʶ���ʵ�������������������ּ�����ѧ�����������ɡ��ܽ�����������������ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԣ�Ҳ����������ѧ���������������ͳ���˼ά������

��ϰ��ϵ�д�
 ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
�����Ŀ

 ��
�� ����ԭ
����ԭ �ķ���Ҳ�������������������Ⱦ�����磺
�ķ���Ҳ�������������������Ⱦ�����磺


 2NH3��g����H����92kJ/mol����ʾ������2 mol NH3ʱ�ų�92.2KJ���ȣ����йؼ��ܣ�N��N��945.6kJ/mol ��N-H��391.0kJ/mol����H��H����Ϊ KJ/mol
2NH3��g����H����92kJ/mol����ʾ������2 mol NH3ʱ�ų�92.2KJ���ȣ����йؼ��ܣ�N��N��945.6kJ/mol ��N-H��391.0kJ/mol����H��H����Ϊ KJ/mol 4CO2(g) + 2H2O(l) + 2600 kJ
4CO2(g) + 2H2O(l) + 2600 kJ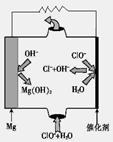
 ��
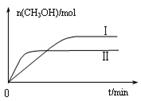
�� 
 CH3OH(g) +H2O(g) ��H
CH3OH(g) +H2O(g) ��H