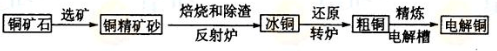
��Ŀ����
ijͬѧ��̽���ϸɵ���ڵĺ�ɫ�����������ʱ��������ͼ��ʾʵ�飺
���Ľ̲Ŀ�֪����ͨп�̵�صĺ�ɫ������Ҫ�ɷ�ΪMnO2��NH4Cl��ZnCl2�����ʡ���ش��������⣺
��1�������ٵ������� ��
��2����������������ʱ������Ҫ�����оƾ��ơ��������� �������Ǻ����żܣ����������������еĺ�ɫ����ʱ������һ����ɫ��ζ��ʹ����ʯ��ˮ����ǵ����壬�ɴ��Ʋ�����ǰ�������д��ڵ������� ��
��3���������ܵ��Թ��м�����������պ����ú�ɫ���壬�Թ���Ѹ�ٲ�����ʹ�����ǵ�ľ����ȼ�����壬�ݴ˿ɳ����϶����պ�ĺ�ɫ����Ϊ , ��������صĻ�ѧ����ʽΪ ��
��4����ͬѧҪ����Һ�ijɷֽ��м��飬��ȷ���Ƿ���NH4+����ͬѧȡ������Һ���Թ��� ����д������������֤ʵ��Һ�к���NH4+��
��5����ͬѧ�����ڷϾɸɵ���л��յ�ZnƬ��ʯī�缫�����һ��ԭ���ʵ�飬�Ƚ�ͭ��п�Ľ������ǿ����
���ò��ϣ�ͭƬ��пƬ��ʯī�缫��ϡ���ᡢCuSO4��Һ��ZnSO4��Һ��
����������ֱ����Դ�������ơ����ߡ��ձ����Թܡ����ŵ���ѧ��ѧ������ҩƷ������
�뻭��ʵ��װ��ͼ��������Ӧ��ע��ͬʱд����������ʽ ��
��1���ܽ�
��2��������C��̼��
��3���������̣�MnO2�� 2H2O2 2H2O+O2��
2H2O+O2��
��4������Ũ����������Һ�����ȣ�����ʪ�ĺ�ɫʯ����ֽ�����Թܿڸ�������ֽ����ɫ
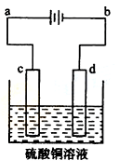
��5��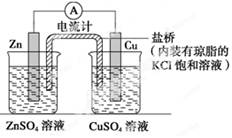
��������ʽ��Cu2+ + 2e- = Cu
����

 �ǻ�С��ϰϵ�д�
�ǻ�С��ϰϵ�д�����ʵ�鷽������������( )
| A����ȥ�����к��е���ϩ��������ͨ����ˮ�У�ϴ�� |
| B�����������Ƿ�ˮ�⣺ȡ1mL 20%��������Һ����3��5��ϡ���ᡣˮԡ����5min��ȡ������Һ��������������Һ����ҺpH�����ԣ��ټ����������Ʊ���Cu(OH)2������3��5 min���۲�ʵ������ |
| C����ȥ���������е��������Ũ������Ҵ������� |
| D������ϩ�ͱ�����������Ȼ�̼��Һ�ֱ�μӵ�������ϩ�ͱ��� |
����ʽΪC4H9Cl ��ͬ���칹�干�У������������칹��
| A��4 | B��5 | C��6 | D��7 |