��Ŀ����
����Ŀ���������Ǻϳ�������(SO2Cl2)�ij��÷�����ʵ���Һϳɵ����ȵķ�Ӧ��ʵ��װ��������ʾ:SO2(g)+Cl2(g) ![]() SO2Cl2(1) ��H=-97.3KJ/mol��
SO2Cl2(1) ��H=-97.3KJ/mol��

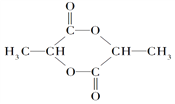
��֪: һ�������£�������Ϊ��ɫ���壬�۵�Ϊ-541�����е�Ϊ69.1��,�ڳ�ʪ�����л�����������100�����Ͽ�ʼ�ֽ⣬���ɶ�����������������ڷ��û�ֽ⡣
��ش���������:
��1��װ�ü��У�70%���������ֵ�����Ϊ_________��
��2����������ʢ�ŵ��Լ���Ҫ�������������Լ�����ʵ���______(������)��ʵ��ʱ�����������зų����Լ��IJ���������_________��
��3����װ�ø��е�ҩƷ����ʵ���________(����ĸ);
a.���������� b. ��ʯ�� c.��ˮ�Ȼ��� d.�轺
��װ�ñ����������г�ˮ����_______(����)
��4������̿��������____����ȱ��װ���ҺͶ�(��ʢ��Ũ����)����ʪ�������Ͷ�����������Ӧ�Ļ�ѧ����ʽ��________��
��5��Ϊ��߱�ʵ��������ȵIJ��ʣ���ʵ������л���Ҫע�����______��
a.���������ٶȣ��������˿�
b.��֤װ�õ�����������
c.����װ�ñ���ͨ��һ�����壬�ų���������ͨ����һ������
d.��ͨ���壬��ͨ����ˮ
���𰸡� ����(��ǿ���ԡ��ѻӷ���) ����ʳ��ˮ ��Һ©���ϿڵIJ�����(����ʹ�������ϵİ�����©���ھ��ϵ�С��)������ת�¿ڵĻ���ʹҺ������ b b ���� SO2+C12+2H2O=H2SO4+2HCl abc
��������������Ҫ������ڡ��������ϳ������ȡ������ۡ�
��1��װ�ü��У�70%���������ֵ�����Ϊ����(��ǿ���ԡ��ѻӷ���)��
��2����������ʢ�ŵ��Լ���Ҫ�������������������ڱ���ʳ��ˮ�����Լ�����ʵ��DZ���ʳ��ˮ��ʵ��ʱ�����������зų����Լ��IJ��������Ǵ�Һ©���ϿڵIJ�����(����ʹ�������ϵİ��ۻ�С����©���ھ��ϵ�С��)������ת�¿ڵĻ���ʹҺ��������
��3����װ�ø��е�ҩƷ����������������ϻ����Ǽ�������b��a��c��d��Ч��
����������ˮ�������ǡ��½��ϳ���������װ�ñ����������г�ˮ����b��
��4������̿�������Ǵ�������ȱ��װ���ҺͶ�(��ʢ��Ũ����)����ʪ�������Ͷ�����������Ӧ�Ļ�ѧ����ʽ��SO2+C12+2H2O=H2SO4+2HCl��
��5��a.��ʹ��Ӧ��ֽ��У�b.��ֹ������ʧ����Ⱦ������c.��ֹ��ʪ�����������ţ�d.Ӧ����ͨ����ˮ����ͨ���壬�Է�������������ʧ����ѡabc��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д�