��Ŀ����
�ýྻ���ձ�ȡ��������ˮ���þƾ��Ƽ��������ڣ����ձ�����μ���5��6�α���FeCl3��Һ��
��1���ù��̵Ļ�ѧ����ʽΪ
��2����ƽϼķ����жϽ������Ʊ��ɹ�
��3����ý�������μ���4mol/L�����ᣬ�����һϵ�б仯��
a���ȳ���
�����������Һ��ɺ��ɫ��ֹͣ����
�����������Һ��ɺ��ɫ��ֹͣ����
��������������������Ƶ�Fe��OH��3���壮��1���ù��̵Ļ�ѧ����ʽΪ
FeCl3+3H2O�TFe��OH��3�����壩+3HCl
FeCl3+3H2O�TFe��OH��3�����壩+3HCl
����2����ƽϼķ����жϽ������Ʊ��ɹ�
��һ���������䣬�ܲ���һ��������ͨ·
��һ���������䣬�ܲ���һ��������ͨ·
����3����ý�������μ���4mol/L�����ᣬ�����һϵ�б仯��
a���ȳ���
���ɫ����
���ɫ����
��b����������ܽ�
�����ܽ�
��������Ӧ�����ӷ���ʽ��Fe��OH��3+3H+=Fe3++3H2O
Fe��OH��3+3H+=Fe3++3H2O
��������ȡ��������ˮ�����������ں����ˮ�е��뼸��FeCl3������Һ�������������Һ��ɺ��ɫ�������Ƶ������������壻
��1���Ʊ������ԭ��������������ˮ�����������������壻
��2�������ж����ЧӦ����һ���������䣬�ܲ���һ��������ͨ·��
��3��������������۳��������������������������������ᣬ�����Ȼ�����ˮ��
��1���Ʊ������ԭ��������������ˮ�����������������壻
��2�������ж����ЧӦ����һ���������䣬�ܲ���һ��������ͨ·��
��3��������������۳��������������������������������ᣬ�����Ȼ�����ˮ��
����⣺ȡ��������ˮ�����������ں����ˮ�е���5��6��FeCl3������Һ�������������Һ��ɺ��ɫ�������Ƶ������������壬
�ʴ�Ϊ�������������Һ��ɺ��ɫ��ֹͣ���ȣ�
��1���Ʊ������ԭ��������������ˮ�����������������壬��Ӧ�Ļ�ѧ����ʽΪFeCl3+3H2O�TFe��OH��3�����壩+3HCl��
�ʴ�Ϊ��FeCl3+3H2O�TFe��OH��3�����壩+3HCl��
��2��������������Һ�Ĺؼ����ڣ���Һ����ֱ��С��1nm����������ֱ����С��1nm��100nm������Һ�����������ЧӦ�����������������ܲ��������ЧӦ��
�ʴ�Ϊ����һ���������䣬�ܲ���һ��������ͨ·���ʴ�Ϊ����һ���������䣬�ܲ���һ��������ͨ·��
��3��������������۳��������������������������������ᣬ�ᷢ������кͷ�Ӧ�������Ȼ�����ˮ���ʴ�Ϊ�����ɫ�����������ܽ⣻Fe��OH��3+3H+=Fe3++3H2O��
�ʴ�Ϊ�������������Һ��ɺ��ɫ��ֹͣ���ȣ�
��1���Ʊ������ԭ��������������ˮ�����������������壬��Ӧ�Ļ�ѧ����ʽΪFeCl3+3H2O�TFe��OH��3�����壩+3HCl��
�ʴ�Ϊ��FeCl3+3H2O�TFe��OH��3�����壩+3HCl��
��2��������������Һ�Ĺؼ����ڣ���Һ����ֱ��С��1nm����������ֱ����С��1nm��100nm������Һ�����������ЧӦ�����������������ܲ��������ЧӦ��
�ʴ�Ϊ����һ���������䣬�ܲ���һ��������ͨ·���ʴ�Ϊ����һ���������䣬�ܲ���һ��������ͨ·��
��3��������������۳��������������������������������ᣬ�ᷢ������кͷ�Ӧ�������Ȼ�����ˮ���ʴ�Ϊ�����ɫ�����������ܽ⣻Fe��OH��3+3H+=Fe3++3H2O��
���������⿼�齺����Ʊ�֪ʶ���ѶȲ���ע����������������Ʊ�����ʽ�dz������������ݣ�����ľ۳��Լ��������ܽ�һֱ�Ǿ���һ���ۺ��Ե��ȵ㣮

��ϰ��ϵ�д�
�����Ŀ
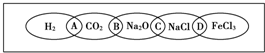 ��ͼΪһ��������ͼ����С����ͼ���Ϸֱ�д��H2��CO2��Na2O��NaCl��FeCl3�������ʣ�ͼ���������������ʾ��ɹ���Ϊһ�࣬�ཻ����A��B��C��DΪ����Ӧ�ķ������ݴ��ţ���ش��������⣺
��ͼΪһ��������ͼ����С����ͼ���Ϸֱ�д��H2��CO2��Na2O��NaCl��FeCl3�������ʣ�ͼ���������������ʾ��ɹ���Ϊһ�࣬�ཻ����A��B��C��DΪ����Ӧ�ķ������ݴ��ţ���ش��������⣺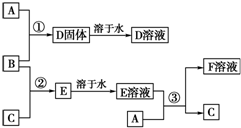 �����£�����A��B��C�ֱ�Ϊ���塢����ɫ���塢��ɫ���壬�ں��ʵķ�Ӧ�����£����ǿ�����ͼ��ʾ���з�Ӧ����֪E��Һ����ɫ�ģ�F��Һ��dz��ɫ�ģ���ش�
�����£�����A��B��C�ֱ�Ϊ���塢����ɫ���塢��ɫ���壬�ں��ʵķ�Ӧ�����£����ǿ�����ͼ��ʾ���з�Ӧ����֪E��Һ����ɫ�ģ�F��Һ��dz��ɫ�ģ���ش�