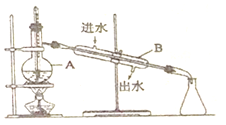
��Ŀ����
����Ŀ�������Լ�ƿ��ǩ�ϵ����ݣ�
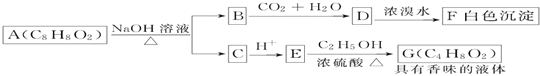
���㣺
��1������������ʵ���Ũ����________mol��L��1��
��2��ij��ѧ��ȤС������������ʵ�ʵ��̽��ʱ����Ҫ90 mL 4.6 mol��L��1��ϡ���ᣬ������ϡ����ʱ��Ҫȡ________mL�ĸ��������ʱ��ѡ�õ�������Ҫ����Ͳ���ձ�����������_______��_______���ں�������д��ȱ���������ƣ�����������ϡ������Һʱ���ݵIJ���Ϊ ___________ ��
��3�����������������ˮ��Ϻ�������Һ�����ʵ����ʵ���Ũ��________9.2 mol��L��1��(����ڻ���ڻ�С��)
��4�����ƹ����У����в�����ʹ���Ƶ�ϡ������ҺŨ��ƫ�ߵ��� ______������ţ���
����ȡŨ�������Ͳ������ˮϴ��2��3�Σ�����ϴ��Һת������ƿ
������ƿʹ��ʱδ����
���ܽ��δ����ȴ����Һ������
�ܶ���ʱ��С������������ˮ�ε�ƿ��
������Ͳ��ȡŨ����ʱ���Ӷ���
���𰸡�18.4 25.0 100ml����ƿ ��ͷ�ι� ������ˮע������ƿ��Һ��������ƿ���̶�����1~2cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ����̶������� С�� �٢ۢ�
��������
��1������c=1000���ѡ���/M���м�����
��2��ʵ����û��90mL����ƿ��Ӧѡ��100mL������ƿ������ϡ�Ͷ��ɼ�������Ũ��������������������Һʱ����Ҫ�������н��������������Һ�ж��ݵľ������������н����
��3��Ũ�����Ũ��Խ���ܶ�Խ��������c=n/V��m����Һ��=��V���з�����
��4������c=n/V���з�������������n�����V��С����������Һ��Ũ�Ⱦ�������
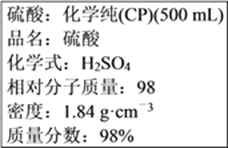
(1)��ŨH2SO4�����ʵ���Ũ��=1000���ѡ���/M=1000��1.84��98%/98=18.4 mol��L��1��
��ˣ�������ȷ������18.4��
��2��ʵ����û��90 mL����ƿ��Ӧѡ��100mL������ƿ������ϡ�Ͷ���,��ҪŨ��������100mL��4.6/18.4 mol��L��1=25.0mL������ʱ��ѡ�õ�������Ҫ����Ͳ���ձ�����������100ml����ƿ����ͷ�ιܣ���������ϡ������Һʱ���ݵIJ���Ϊ��������ˮע������ƿ��Һ��������ƿ���̶�����1~2cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ����̶������У�
�������������������25.0��100ml����ƿ����ͷ�ιܣ� ������ˮע������ƿ��Һ��������ƿ���̶�����1~2cmʱ�����ý�ͷ�ιܵμ�����ˮ��Һ����̶������С�
��3�����������ˮ��������Ϊm����Ϻ���Һ���ܶ�Ϊ��1��18.4 mol��L��1Ũ������ܶ�Ϊ������������w=cM/1000������Ϻ���Һ��Ũ��c=n/V=[(m����/M)]/[2m/1000��1]��������֪�����Ϊ��c=(18.4/2)��(��1/��)=9.2��1/�ѣ�Ũ������ܶ���Ũ�ȵĹ�ϵΪ��Ũ��Խ���ܶ�Խ����>��1����cС��9.2mol/L��
�������������������С�ڡ�
��4������ȡŨ�������Ͳ������ˮϴ��2��3�Σ�����ϴ��Һת������ƿ���������������������ʹ���Ƶ�ϡ������ҺŨ��ƫ�ߣ���ȷ��
������ƿʹ��ʱδ���������Ҫ��ˮ���ݣ��Խ����Ӱ�죬����
���ܽ��δ����ȴ����Һ�����ݣ�����ȴ����Һ���ƫС��Ũ��ƫ�ߣ���ȷ��
�ܶ���ʱ��С������������ˮ�ε�ƿ�⣬��Ϊ��ʧ��������ˮ�����������ʣ����ݶ��ݵ�ԭ���Լ�����ˮ���̶��ߣ���������Һ��Ũ����Ӱ�죬����
������Ͳ��ȡŨ����ʱ���Ӷ�������ȡŨ����ƫ�࣬���ʵ���ƫ��Ũ��ƫ�ߣ���ȷ��
������Ϸ�����֪���٢ۢ���ȷ��
��������������ѡ�٢ۢݡ�

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�����Ŀ��ij��Ӧ�����Ϊ5L�ĺ����ܱյľ��������н��У������ʵ�����ʱ��ı仯�������ͼ��ʾ����֪A��B��C��Ϊ���壩��

��1���÷�Ӧ�Ļ�ѧ����ʽΪ_______��
��2����Ӧ��ʼ��2����ʱ��B��ƽ����Ӧ����Ϊ_______��
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����_______
A���ԣ�A����2�ԣ�B�� B�������������ܶȲ���
C��v�棨A����������C�� D������ֵ����ʵ������
E. ��������ƽ����Է����������ٸı��״̬
��4����ͼ���ƽ��ʱ����ת����Ϊ_______��
��5��Ϊ���о�����ͭ�����������������ʵ�Ӱ�죬ijͬѧ���������һϵ�е�ʵ�飺�����������Ļ����Һ�ֱ���뵽6��ʢ�й���Zn���������У��ռ����������壬��¼�����ͬ�������������ʱ�䡣
ʵ�� �����Һ | A | B | C | D | E | F |
4 mol/L H2SO4(mL) | 30 | V1 | V2 | V3 | V4 | V5 |
����CuSO4��Һ(mL) | 0 | 0.5 | 2.5 | 5 | V6 | 20 |
H2O(mL) | V7 | V8 | V9 | V10 | 10 | 0 |
������ɴ�ʵ����ƣ����У�V1=_______��V6=_______��
�ڸ�ͬѧ���ó��Ľ���Ϊ������������CuSO4��Һʱ���������������ʻ�����ߣ����������CuSO4��Һ����һ����ʱ���������������ʷ������½���������������������½�����Ҫԭ��_______��