��Ŀ����
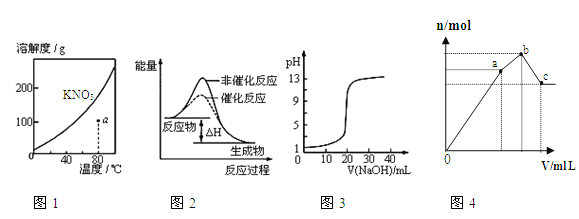
����Ŀ����Ʋ����Ĺ�ҵ��ˮ���е��ؽ������ӣ�Cu2+��Ni2+�ȣ���CN�γ����Գ�ȥ�������õ�ⷨ�Ʊ��������ƣ�Na2FeO4)������ˮ��Ŀǰ�Ƚ��Ƚ��ķ�������֪�軯���ж��ӷ������װ����ͼ��ʾ������ѡ����ȷ���ǣ� ��
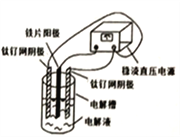
A. ��Ƭ���ӵ�Դ�ĸ���
B. ���ʱ��ÿ����lmolFeO42-��ת�Ƶ���3mol
C. CN��������CO2��N2��Cu2+��Ni2+�γ����ܼ����ȥ
D. ��֪HFeO4-�������Ա�FeO42-��ǿ����pH<7ʱ������ˮЧ�ʸ���
���𰸡�C
��������A. ��Ƭ�����������ӵ�Դ��������A����B. ��Ԫ�ػ��ϼ۴�0�����ߵ�+6�ۣ���˵��ʱ��ÿ����lmolFeO42-��ת�Ƶ���6mol��B������C. �������ξ���ǿ�����ԣ���CN��������CO2��N2�������������ӷŵ���������������Cu2+��Ni2+�γ����ܼ����ȥ��C��ȷ��D. pH<7ʱ��������HCN����Ⱦ������D����ѡC��

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�����Ŀ���±���ʾʵ�飬����ͽ��۾���ȷ���ǣ� ��
ѡ�� | ʵ�� | ���� | ���� |
A | ��Ũ�Ⱦ�Ϊ0.lmol��L-1NaCl��NaI�����Һ�еμ�����AgNO3��Һ | ���ֻ�ɫ���� | Ksp(AgCl)>Ksp(AgI) |
B | �����£��ⶨ�����ʵ���Ũ�ȵ�Na2CO3��Na2SO3��Һ��pHֵ | ǰ�ߵ� pHֵ�Ⱥ��ߵĴ� | �ǽ����ԣ�S>C |
C | ��ij��Һ�еμ��������� | ��Һ�г��������ݺ͵���ɫ���� | ��Һ�к���S2-�� SO32- |
D | ��FeCl3��KSCN�����Һ�У���������KCl�Ĺ��� | ��Һ��ɫ��dz | FeCl3 +KSCN |
A. A B. B C. C D. D
����Ŀ���˰�ȫ���������Ⱦ��������������˴���Ĺ㷺���ӡ��ڱ�ը�ĺ˵�վ��Χ���з��������ʵ�һ131���һ 137���⡪131�������������룬���ܻ�������״�ٵȼ�����
(l)Cs(�)�ļ۵��ӵĵ����Ų�ʽΪ6s1�����ͬ�����ǰ�����ڣ������������ڣ�������Ԫ��X��Y��Z�ĵ��������±���
Ԫ�ش��� | X | Y | Z |
��һ�����ܣ�kJ��mol-1) | 520 | 496 | 419 |
��������Ԫ��X��Y��Z��Ԫ�ط��ŷֱ�Ϊ_________����̬Zԭ�ӵĺ�������Ų�ʽΪ______��X�γɵĵ��ʾ����к��еĻ�ѧ��������_________________��
(2)F��Iͬ���壬BeF2��H2O����������ԭ�ӹ��ɵĹ��ۻ�������ӣ����߷����е�����ԭ��Be��O���ӻ���ʽ�ֱ�Ϊ______��______��BeF2���ӵ����幹����____________��H2O���ӵ����幹����________________��
(3)���ͬ������Ⱦ��к�ǿ�Ļ����ԣ����γɴ����ĺ��Ȼ����BC13������B��C1���ļ���Ϊ__________________��
(4)131I2����ľ����ṹ��ͼ����ʾ���þ����к���____��131I2���ӣ�KI�ľ����ṹ��ͼ����ʾ��ÿ��K+����______��I-��

(5)KI������ܶ�Ϊ��g cm 3��K��I��Ħ�������ֱ�ΪMK g mol-1��M1g mol-1��ԭ�Ӱ뾶�ֱ�ΪrKpm��r1 pm�������ӵ�����ֵΪNA����KI������ԭ�ӵ����ռ��������İٷ���Ϊ_____________��