��Ŀ����
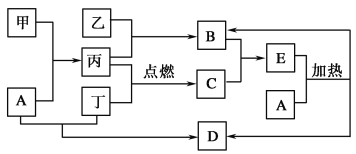
����Ŀ�����п�ͼ��ʾ������ת����ϵ�У������ճ������г����Ľ������ҡ��������dz��������嵥�ʡ�����B������C�������������İ�������E��A��ǿ�D�Ǻ�ˮ��Ũ����ߵ���(���ַ�Ӧ��������Pˮ����ȥ)��
��ش��������⣺
��1��д�����������B����Ӧ������________________��
��2��д����A��Һ��Ӧ�����ӷ���ʽ��______________��
��3������E�е�������ʱ��ȡ����E���Թ��У� ��֤��E���и������ӡ�
��4��д��ʵ������ȡB�Ļ�ѧ����ʽ��________________��
��5��B������;��_______________��_______________��_______________��
��6����������ҵ��ij���ԭ�ϣ�д����B�Ƹ�������еĻ�ѧ����ʽ��
______________________�� ��________________��
���𰸡�(1)���¸�ѹ����(2)2Al��2OH����2H2O��2AlO2����3H2��
(3)����NaOH��Һ����������ʪ���ʯ����ֽ�����Թܿ�����ֽ����
(4)2NH4Cl��Ca(OH)2![]() CaCl2��2NH3����2H2O(5)��������ƻ�����������
CaCl2��2NH3����2H2O(5)��������ƻ�����������
��6��4NH3��5O2![]() 4NO��6H2O 2NO��O2��2NO2 3NO2��H2O��2HNO3��NO
4NO��6H2O 2NO��O2��2NO2 3NO2��H2O��2HNO3��NO
��������
�������������B������C�������������İ�������E����˵��EӦ�����Ȼ�泥�B��CӦ�����Ȼ���Ͱ��������Ͷ���ȼ����C������C���Ȼ��⣬�������������������������ǵ�����B�ǰ�����A��ǿ�D�Ǻ�ˮ��Ũ����ߵ��Σ���D���Ȼ��ƣ�����A�����������ơ�������������Һ��Ӧ�������������Լ��ǵ���Al��
��1���ϳɰ�����Ӧ�����Ǹ��¸�ѹ������
��2����A��Һ��Ӧ�����ӷ���ʽΪ2Al��2OH����2H2O��2AlO2����3H2����
��3������E�е�������ʱ��ȡ����E���Թ��У�����NaOH��Һ����������ʪ���ʯ����ֽ�����Թܿ�����ֽ��������֤��E���и������ӡ�
��4��ʵ������ȡ�����Ļ�ѧ����ʽΪ2NH4Cl��Ca(OH)2![]() CaCl2��2NH3����2H2O��
CaCl2��2NH3����2H2O��
��5�������ij�����;����������ƻ����������ᡣ
��6�����������������еĻ�ѧ����ʽΪ4NH3��5O2![]() 4NO��6H2O��2NO��O2��2NO2��3NO2��H2O��2HNO3��NO��
4NO��6H2O��2NO��O2��2NO2��3NO2��H2O��2HNO3��NO��