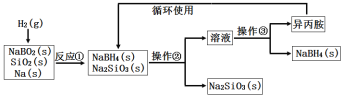
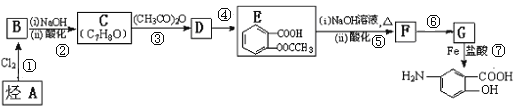
��Ŀ����
����Ŀ����1����֪����2H2��g��+O2��g��=2H2O��l����H1=-571.6kJmol-1
��2CO��g��+O2��g��=2CO2��g����H2=-566.0kJmol-1
��CO��g��+2H2��g��![]() CH3OH��g����H3=-90.8kJmol-1
CH3OH��g����H3=-90.8kJmol-1
����״�������ȼ������H�� ��
��2�����ڿ��淴ӦC��S��+H2O��g��![]() CO��g��+H2��g����ƽ�ⳣ������ʽΪ �����жϸ÷�Ӧһ���ﵽ��ѧƽ��״̬�������� ������ѡ������
CO��g��+H2��g����ƽ�ⳣ������ʽΪ �����жϸ÷�Ӧһ���ﵽ��ѧƽ��״̬�������� ������ѡ������
A�������������ƽ����Է�����������ʱ����仯
B��v����H2O��=v����H2��
C��������������ܶȲ���ʱ����仯
D������������������ʱ����仯
E������n mol H2��ͬʱ����n mol CO
��3��ij��ѧ��ȤС��̽����������Ժϳɼ״���Ӧ��Ӱ�졣
CO��g��+2H2��g��![]() CH3OH��g����H=-91kJmol-1
CH3OH��g����H=-91kJmol-1
����300��ʱ�������Ϊ1L���ܱ������м���2mol H2��1mol CO��CO��CH3OH��g����Ũ����ʱ��仯��ͼ��ʾ��
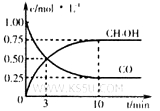
��ӷ�Ӧ��ʼ������ƽ�⣬v��H2��Ϊ ��
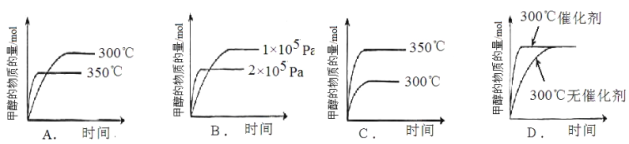
����������������ʱ��ֻ�ı����е�һ����������С��ͬѧ����ʵ���������ͼ����������ʵ������� �����������
���𰸡�
��1����-763.8 kJ/mol��
��2��K=![]() ��ABC��
��ABC��
��3����0.15mol/Lmin����AD��
��������
�����������1����2H2��g��+O2��g��=2H2O��l����H1=-571.6kJmol-1��
��2CO��g��+O2��g��=2CO2��g����H2=-566.0kJmol-1��
��CO��g��+2H2��g��![]() CH3OH��g����H3=-90.8kJmol-1��
CH3OH��g����H3=-90.8kJmol-1��
���ݸ�˹����֪����+�ڡ�![]() -������CH3OH��g��+
-������CH3OH��g��+![]() O2��g��=CO2��g��+2H2O��l����
O2��g��=CO2��g��+2H2O��l����
��H=-��571.6+![]() ��566.0-90.8��kJ/mol=-763.8kJ/mol���ʴ�Ϊ��-763.8kJ/mol��
��566.0-90.8��kJ/mol=-763.8kJ/mol���ʴ�Ϊ��-763.8kJ/mol��
��2�����淴ӦC��S��+H2O��g��![]() CO��g��+H2��g����ƽ�ⳣ������ʽK=
CO��g��+H2��g����ƽ�ⳣ������ʽK=![]() ��A����Ӧǰ��������������ͬ�������������ƽ����Է����������ϼ�С����ƽ����Է�����������ʱ��仯���ﵽƽ��״̬����A��ȷ��B��������H2O��=������H2��ʱ�������Ũ�Ȳ��ٱ仯����Ӧ�ﵽƽ��״̬����B��ȷ��C����Ӧ��CΪ���壬��Ӧǰ�������������������ݻ����䣬��������
��A����Ӧǰ��������������ͬ�������������ƽ����Է����������ϼ�С����ƽ����Է�����������ʱ��仯���ﵽƽ��״̬����A��ȷ��B��������H2O��=������H2��ʱ�������Ũ�Ȳ��ٱ仯����Ӧ�ﵽƽ��״̬����B��ȷ��C����Ӧ��CΪ���壬��Ӧǰ�������������������ݻ����䣬��������![]() ��֪��������������ܶȲ�������������������ܶȲ���ʱ��仯ʱ��˵����Ӧ�ﵽƽ��״̬����C��ȷ��D�����������غ㶨�ɿ�֪����������������ʼ���ղ���ʱ����仯����D����E������n mol H2��ͬʱ����n mol CO����ʾ�Ķ����淴Ӧ�����ж����淴Ӧ�����Ƿ���ȣ���E���ʴ�Ϊ��K=
��֪��������������ܶȲ�������������������ܶȲ���ʱ��仯ʱ��˵����Ӧ�ﵽƽ��״̬����C��ȷ��D�����������غ㶨�ɿ�֪����������������ʼ���ղ���ʱ����仯����D����E������n mol H2��ͬʱ����n mol CO����ʾ�Ķ����淴Ӧ�����ж����淴Ӧ�����Ƿ���ȣ���E���ʴ�Ϊ��K=![]() ��A BC��
��A BC��
��3������300��ʱ�������Ϊ1L���ܱ������м���2mol H2��1mol CO��CO��CH3OH��g����Ũ����ʱ��仯��ͼ2��ʾ����ӷ�Ӧ��ʼ������ƽ�⣬ƽ��Ũ��c��CH3OH��=0.75mol/L��c��CO��=0.25mol/L���ﵽƽ��ʱ��Ϊ10min����
CO��g��+2H2��g��![]() CH3OH��g��
CH3OH��g��
��ʼ����mol/L�� 1 2 0
�仯����mol/L�� 0.75 1.5 0.75
ƽ������mol/L�� 0.25 0.5 0.75
v��H2��=![]() =0.15mol/Lmin���ʴ�Ϊ��0.15mol/Lmin��
=0.15mol/Lmin���ʴ�Ϊ��0.15mol/Lmin��
��CO��g��+2H2��g��![]() CH3OH��g����H=-91kJmol-1����Ӧ�Ƿ��ȷ�Ӧ��A��ͼ�����ȹ���ƽ���¶ȸߣ��¶�Խ�ߣ�ƽ��������У��״����ʵ�����С����A���ϣ�B��ͼ�����ȹ���ƽ��ѹǿ��Ӧ�����������С�ķ�Ӧ��ѹǿ����ƽ��������У��״����ʵ�������B�����ϣ�C������ƽ���ƶ�ԭ����֪�¶�Խ�ߣ�ƽ��������У��״����ʵ�����С��ͼ���ϣ���C�����ϣ�D����������ӿ췴Ӧ���ʣ����̴ﵽƽ���ʱ�䣬���ﵽƽ��״̬��ͬ����D���ϣ��ʴ�Ϊ��AD��
CH3OH��g����H=-91kJmol-1����Ӧ�Ƿ��ȷ�Ӧ��A��ͼ�����ȹ���ƽ���¶ȸߣ��¶�Խ�ߣ�ƽ��������У��״����ʵ�����С����A���ϣ�B��ͼ�����ȹ���ƽ��ѹǿ��Ӧ�����������С�ķ�Ӧ��ѹǿ����ƽ��������У��״����ʵ�������B�����ϣ�C������ƽ���ƶ�ԭ����֪�¶�Խ�ߣ�ƽ��������У��״����ʵ�����С��ͼ���ϣ���C�����ϣ�D����������ӿ췴Ӧ���ʣ����̴ﵽƽ���ʱ�䣬���ﵽƽ��״̬��ͬ����D���ϣ��ʴ�Ϊ��AD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�