��Ŀ����
2����1���뽫�����������ʣ�KBrO3��Br2��I2��KI��K2SO4�ֱ��������ж�Ӧ�ĺ����ϣ����һ��δ��ƽ�Ļ�ѧ����ʽ��KBrO3+KI+H2SO4��Br2+I2+K2SO4+KBr+H2O����2���÷�Ӧ�е�����������I2������ԭ����Br2��KBr�У���д��ѧʽ����BrԪ�أ���дԪ�ط��ţ���
��3������÷�Ӧ����ʽ��I2 �� KBr��ϵ���ֱ���16��2����
��Br2��ϵ����2��
���뽫��Ӧ��Ļ�ѧʽ����ƽ���ϵ������������Ӧ��λ���У�
6KBrO3+32KI+H2SO4��16I2+2Br2+18K2SO4+2KBr+18H2O
����ת�Ƶ�������Ϊ10mol����Ӧ��I2�����ʵ���Ϊ5mol��
���� ��1���ɸ��������ʿ�֪��BrԪ�صĻ��ϼ۽��ͣ��������뻹ԭ��KI����Ӧ���������Ϊ�����
��2������Ԫ�ػ��ϼ����ߵIJ���Ϊ�����������Ԫ�ػ��ϼ۽��͵IJ���Ϊ��ԭ���
��3������I2ǰ�Ļ�ѧ��������16��KBrǰ�Ļ�ѧ��������2�����ݵ���ת���غ����Br2�Ļ�ѧ��������
�ڸ���IԪ���غ��֪��KI�Ļ�ѧ��������32������BrԪ���غ�ȷ��KBrO3�Ļ�ѧ����������Kԭ���غ��֪K2SO4�Ļ�ѧ������������������غ�ȷ��H2SO4�Ļ�ѧ���������ٸ���HԪ���غ�ȷ��H2O��ϵ����
�۷�Ӧ��KI��I2��IԪ�ػ��ϼ���-1������Ϊ0�ۣ���������ֻ��I2�����ݵ���ע���غ��������I2�����ʵ�����
��� �⣺��1������BrԪ�ط�Ӧ�ϼ۽��Ϳ�֪���������뻹ԭ��KI����Ӧ���������I2��Br2��K2SO4��KBrΪ�����֪��ӦΪKBrO3+KI+H2SO4��Br2+I2+K2SO4+KBr+H2O��
�ʴ�Ϊ��KBrO3��KI��Br2��I2��K2SO4��
��2�����ݷ���ʽ��֪��IԪ�صĻ��ϼ����߶�Ӧ����Ϊ��I2��BrԪ�صĻ��ϼ۽��Ͷ�Ӧ����Ϊ��Br2��KBr���ʴ�Ϊ��I2��Br2��KBr��Br��
��3������I2�Ļ�ѧ��������16��KBr�Ļ�ѧ��������2����IԪ�ع�ʧ����19mol��2=32 mol��KBr�Ļ�ѧ��������2���õ���2mol��[5-��-1��]=12mol����KBrO3��Br2Ҫ�õ���20mol����Br2�Ļ�ѧ������Ϊ$\frac{20}{2��5-0��}$=2���ʴ�Ϊ��2��
�ڸ���IԪ���غ��֪��KI�Ļ�ѧ��������32����BrԪ���غ�ȷ��KBrO3�Ļ�ѧ������Ϊ2+2��2=6����Kԭ���غ��֪K2SO4�Ļ�ѧ������Ϊ$\frac{6+32-2}{2}$=18������������غ�ȷ��H2SO4�Ļ�ѧ������Ϊ18���ٸ���HԪ���غ�ȷ��H2O��ϵ��Ϊ18������ƽ��ʽΪ��6KBrO3+32KI+18H2SO4=16I2+2Br2+18K2SO4+2KBr+18H2O��
�ʴ�Ϊ��6KBrO3��32KI��16I2+2Br2+18K2SO4+2KBr+18H2O��
�۷�Ӧ��KI��I2��IԪ�ػ��ϼ���-1������Ϊ0�ۣ���������ֻ��I2����ת��10mol��������I2�����ʵ���Ϊ��$\frac{10mol}{2}$=5mol��
�ʴ�Ϊ��5mol��
���� ���⿼��������ԭ��Ӧ��ƽ����ؼ��㣬��Ϥ������ԭ��Ӧ�ĸ����ʧ�����غ�����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

| A�� | ͨ��״����22.4L���� | B�� | 16gˮ | ||
| C�� | NA��H2 | D�� | ��״����22.4Lˮ |
| A�� | �����ж���Һ���� | |
| B�� | Cu��������ȼ�գ�������ɫ����CuCl2 | |
| C�� | Cl2��Ӧ�����κ�ˮ | |
| D�� | ��ҵ�Ͽɽ�H2��Cl2��Ϲ������ϳ�HCl |
�ٲ�����ˮ���Σ�CaCO3��BaSO4�ȣ������������
���ζ���ǿ�����
��0.5mol•L-1������һԪ����������Ũ�ȶ���0.5mol•L-1
��ǿ����Һ��������Ũ��һ������������Һ��������Ũ��
�ݵ������Һ�����ԭ������Һ���������ƶ�����������
�����ڵĵ���ʶ��ܵ��磮
| A�� | �٢ۢݢ� | B�� | �ڢۢܢ� | C�� | ֻ�Т� | D�� | ֻ�Т� |
| A�� | 2Fe3++Fe�T3Fe2+����˵�������ԣ�Fe3+��Fe2+ | |
| B�� | 25�桢pH=0����Һ�У�Al3+��NH4+��NO3-��Fe2+���Դ������� | |
| C�� | 5.6 g����������������Ӧʧȥ�ĵ���Ϊ0.2 mol | |
| D�� | ����������Һ�м������������Һ��Fe2++2H2O2+4H+�TFe3++4H2O |
| A�� |  ��ʾ KNO3 ���ܽ�����ߣ�ͼ�� a ���ʾ����Һͨ�����¿��Եõ� b �� | |
| B�� |  ��ʾijһ���ȷ�Ӧ����ʹ�ô��� E1��E2����H ���ᷢ���ı� | |
| C�� |  ��ʾ�� Na2CO3 �� NaHCO3 �Ļ����Һ�еμ�ϡ����ʱ������ CO2 ����� | |
| D�� |  ��ʾ�� 100mL0.1mol/L �� AlCl3 �� 0.1mol/L �� NH4Cl �����Һ�еμ� 1mol/L �� NaOH ��Һʱ n��Al3+�� �� n��AlO2-���ı仯��� |
| A�� | ԭ���ǹ���һ�����ʵ��� | |
| B�� | ԭ���ǻ�ѧ�仯�е���С�� | |
| C�� | ԭ���ǹ������ʵ�һ���� | |
| D�� | ԭ���DZ������ʻ�ѧ���ʵ���С�� |
| A�� | ±���ǵ��͵ķǽ���Ԫ�أ���˲�������������Ԫ�ػ��� | |
| B�� | ͬ������±��Ԫ�صĵ縺����С | |
| C�� | ±�ظ����ʶ��ܺ�ˮ���ҷ�Ӧ | |
| D�� | ±�ص��ʶ��ܺ�H2��Ӧ������̬�⻯��������浥�������Ե���ǿ����ǿ |
| A�� | �����ϩ��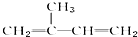 ��������ʵ�����Br2�����ӳɷ�Ӧ ��������ʵ�����Br2�����ӳɷ�Ӧ | |
| B�� | 2-�ȶ�����NaOH�Ҵ���Һ���ȷ�����ȥHCl���ӵķ�Ӧ | |
| C�� | �ױ���һ�������·���������Ӧ����һ�����ױ��ķ�Ӧ | |
| D�� | ���ǻ���������NaHCO3��Һ��Ӧ |