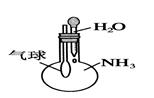
��Ŀ����
�Ա�������ζ�δ֪Ũ�ȵ���������Ϊ�����ж����²�������������ƫ��ƫС�����䡱����
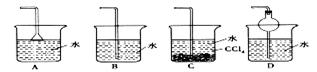
��1���������ζ�ǰ���ӻ�ζ������� �� ��
��2��δ�ñ�Һ��ϴ��ʽ�ζ��� �� ��
��3���ô���Һ��ϴ��ƿ �� ��
��4���ζ�ǰ�ζ��ܼ��������ݣ��ζ������������ʧ �� ��
��5����Һ����ȡ����Һ�����շ�����ƿ����������Һ����ƿ�� �� ��
��6����ʽ�ζ��ܣ�������Һ�ã�����Һ��������ˮϴ����ֱ��ע�����Һ �� ��
��7���ζ������У���ƿ��̫���ң�������Һ�ν��� �� ��
��8����ʼʱ��Һ�ڵζ��̶ܿ������ϣ�δ����� �� ��
��9����ƿ������ˮ��ϴ���������ֱ��ʢ������Һ �� ��
��10���ζ��ӽ��յ�ʱ������������ˮ��ϴ��ƿ�ڱ� �� ��
��1���������ζ�ǰ���ӻ�ζ������� �� ��
��2��δ�ñ�Һ��ϴ��ʽ�ζ��� �� ��
��3���ô���Һ��ϴ��ƿ �� ��
��4���ζ�ǰ�ζ��ܼ��������ݣ��ζ������������ʧ �� ��
��5����Һ����ȡ����Һ�����շ�����ƿ����������Һ����ƿ�� �� ��
��6����ʽ�ζ��ܣ�������Һ�ã�����Һ��������ˮϴ����ֱ��ע�����Һ �� ��
��7���ζ������У���ƿ��̫���ң�������Һ�ν��� �� ��
��8����ʼʱ��Һ�ڵζ��̶ܿ������ϣ�δ����� �� ��
��9����ƿ������ˮ��ϴ���������ֱ��ʢ������Һ �� ��
��10���ζ��ӽ��յ�ʱ������������ˮ��ϴ��ƿ�ڱ� �� ��
��20�֣���ÿ��2�֣���1��ƫ�� ��2��ƫ�� ��3��ƫ�� ��4��ƫ��
��5��ƫС ��6��ƫС ��7��ƫС ��8��ƫС ��9����Ӱ�� ��10����Ӱ��
��5��ƫС ��6��ƫС ��7��ƫС ��8��ƫС ��9����Ӱ�� ��10����Ӱ��
�����������������������Ϊ����C��==C��V��/V������C�ꡢV����Ϊ��ֲ������C��Ĵ�Сȡ����V��Ĵ�С����V�꣺ƫ���ƫС����C��ƫ���ƫС���ݴ˿�֪��
��1���ζ�ǰ���ӣ�����ƫС����ζ������ӣ�����ƫ�����Բⶨ���ƫ�ߡ�
��2��δ�ñ�Һ��ϴ��ʽ�ζ��ܣ������ı�Һ��������ӣ��ⶨ���ƫ�ߡ�
��3���ô���Һ��ϴ��ƿ�������ı�Һ��������ӣ��ⶨ���ƫ�ߡ� ��4���ζ�ǰ�ζ��ܼ��������ݣ��ζ������������ʧ���������ı�Һ��������ӣ��ⶨ���ƫ�ߡ�
��5����Һ����ȡ����Һ�����շ�����ƿ����������Һ����ƿ�⣬�����Һ��������٣����ı�Һ��������٣��ⶨ���ƫ�͡�
��6����ʽ�ζ��ܣ�������Һ�ã�����Һ��������ˮϴ����ֱ��ע�����Һ�����൱����ϡ�ͣ����ı�Һ��������٣��ⶨ���ƫ�͡�
��7���ζ������У���ƿ��̫���ң�������Һ�ν����������ı�Һ��������٣��ⶨ���ƫ�͡�
��8����ʼʱ��Һ�ڵζ��̶ܿ������ϣ�δ����������൱���Ǽ��ٱ�Һ������������ı�Һ��������٣��ⶨ���ƫ�͡�
��9����ƿ������ˮ��ϴ���������ֱ��ʢ������Һ���Բⶨ���û��Ӱ�졣
��10���ζ��ӽ��յ�ʱ������������ˮ��ϴ��ƿ�ڱڣ��Բⶨ���û��Ӱ�졣
�������������е��Ѷȵ����⣬������۽̲ģ����ض�ѧ��ʵ��������������ּ������ѧ��������û���֪ʶ���ʵ�����������������������ѧ������˼ά�����淶�Ͻ���ʵ���������������Ĺؼ�����ȷʵ��ԭ�����Լ���������ԭ��Ȼ��������ü��ɡ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
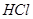 ������
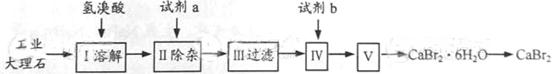
������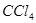 ������ͼ��ʾ������װ���У���������
������ͼ��ʾ������װ���У���������