��Ŀ����
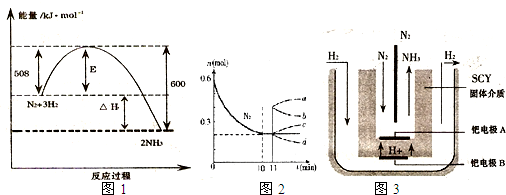
��1���ϳɰ���Ӧ��Ӧ��N2��g��+3H2��g��
2NH3��g��
���ں�����������ϵ��ѹǿ��ƽ��
��2����֪��
CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g������H=-574kJ?mol-1
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g������H=-1160kJ?mol-1
��CH4ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ��
��3����25���£���Ũ�Ⱦ�Ϊ0.1mol?L-1��AlCl3��CuCl2�����Һ�м��������Ũ��ˮ�����õ�����ɫ��Һ�Ͱ�ɫ��������ɫ�����Ļ�ѧʽΪ
��4����25���£���a mol?L-1�İ�ˮ��0.01mol?L-1������������ϣ���Ӧƽ��ʱ��Һ��c��NH4+��=c��Cl-��������Һ��
| ���¡���ѹ | ���� |
���ں�����������ϵ��ѹǿ��ƽ��
����
����
����������ҡ��������ƶ���ʹ�ô�����Ӧ�ġ�H���ı�
���ı�
���������С�����ı䡱������2����֪��
CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g������H=-574kJ?mol-1
CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g������H=-1160kJ?mol-1
��CH4ֱ�ӽ�NO2��ԭΪN2���Ȼ�ѧ����ʽΪ��
CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H=-867kJ?mol-1
CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H=-867kJ?mol-1
����3����25���£���Ũ�Ⱦ�Ϊ0.1mol?L-1��AlCl3��CuCl2�����Һ�м��������Ũ��ˮ�����õ�����ɫ��Һ�Ͱ�ɫ��������ɫ�����Ļ�ѧʽΪ
Al��OH��3
Al��OH��3
�����ɸó��������ӷ���ʽΪAl3++3NH3?H2O=Al��OH��3��+3NH4++3H2O
Al3++3NH3?H2O=Al��OH��3��+3NH4++3H2O
����4����25���£���a mol?L-1�İ�ˮ��0.01mol?L-1������������ϣ���Ӧƽ��ʱ��Һ��c��NH4+��=c��Cl-��������Һ��
��
��
�ԣ���ᡱ������С�������������1������ѹǿ����ѧƽ���������������С�ķ�����У�����ֻ�ܸı䷴Ӧ���ʣ�
��2�����ݸ�˹���������㷴Ӧ���ʱ䣻
��3�����ݰ�ˮ���Ȼ����Լ��Ȼ�ͭ�ķ�Ӧ���ش�
��4�����ݵ���غ����жϣ�
��2�����ݸ�˹���������㷴Ӧ���ʱ䣻
��3�����ݰ�ˮ���Ȼ����Լ��Ȼ�ͭ�ķ�Ӧ���ش�
��4�����ݵ���غ����жϣ�
����⣺��1�����ݻ�ѧƽ���ƶ�ԭ��������ѹǿ����ѧƽ���������������С�ķ�����У��������ں�����������ϵ��ѹǿ��ƽ��N2��g��+3H2��g��
2NH3��g�������ҽ��У�����ֻ�ܸı䷴Ӧ���ʣ�����ı䷴Ӧ����ЧӦ���ʴ�Ϊ�����ң����ı䣻
��2����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g������H=-574kJ?mol-1
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g������H=-1160kJ?mol-1
��Ӧ��+�ڿɵõ�2CH4��g��+4NO2��g���T2N2��g��+2CO2��g��+4H2O�� g ����
��H=��-574kJ?mol-1��+��-1160kJ?mol-1��=-1734kJ?mol-1��
��CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O�� g ����H=-867kJ?mol-1��
�ʴ�Ϊ��CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O�� g ����H=-867kJ?mol-1��
��3��AlCl3��Ũ��ˮ��Ӧ�Ļ�ѧ����ʽΪ��Al3++3NH3?H2O=Al��OH��3��+3NH4++3H2O��
�ʴ�Ϊ��Al��OH��3��Al3++3NH3?H2O=Al��OH��3��+3NH4++3H2O��
��4����a mol?L-1�İ�ˮ��0.01mol?L-1������������ϣ�
���ݵ���غ㣺c��NH4+��+c��H+��=c��Cl-��+c��OH-����c��NH4+��=c��Cl-����c��H+��=c��OH-������Һ�����ԣ�
�ʴ�Ϊ���У�
| ���¡���ѹ |
| ���� |
��2����CH4��g��+4NO2��g��=4NO��g��+CO2��g��+2H2O��g������H=-574kJ?mol-1
��CH4��g��+4NO��g��=2N2��g��+CO2��g��+2H2O��g������H=-1160kJ?mol-1
��Ӧ��+�ڿɵõ�2CH4��g��+4NO2��g���T2N2��g��+2CO2��g��+4H2O�� g ����
��H=��-574kJ?mol-1��+��-1160kJ?mol-1��=-1734kJ?mol-1��
��CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O�� g ����H=-867kJ?mol-1��
�ʴ�Ϊ��CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O�� g ����H=-867kJ?mol-1��
��3��AlCl3��Ũ��ˮ��Ӧ�Ļ�ѧ����ʽΪ��Al3++3NH3?H2O=Al��OH��3��+3NH4++3H2O��
�ʴ�Ϊ��Al��OH��3��Al3++3NH3?H2O=Al��OH��3��+3NH4++3H2O��
��4����a mol?L-1�İ�ˮ��0.01mol?L-1������������ϣ�
���ݵ���غ㣺c��NH4+��+c��H+��=c��Cl-��+c��OH-����c��NH4+��=c��Cl-����c��H+��=c��OH-������Һ�����ԣ�
�ʴ�Ϊ���У�
������������һ���ۺ�֪ʶ��Ŀ������ѧ�������ͽ��������������Ѷ��еȣ�

��ϰ��ϵ�д�
 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�
�����Ŀ
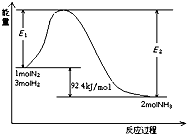 ��һ��������ܱ������У��������»�ѧ��Ӧ��
��һ��������ܱ������У��������»�ѧ��Ӧ��