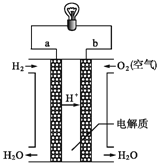
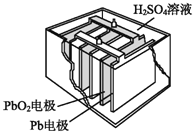
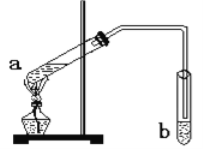
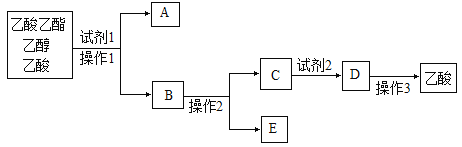
��Ŀ����
����Ŀ������8��Ԫ�ص����ʡ��������±����У��������ڵڶ���������ڡ�
�� | �� | �� | �� | �� | �� | �� | �� | |
ԭ�Ӱ뾶(10��10m) | 0.74 | 1.60 | 1.86 | 1.10 | 0.99 | 1.52 | 0.75 | 1.43 |
�����ͻ��ϼ� | ��2 | ��1 | ��5 | ��7 | ��1 | ��5 | ��3 | |
��2 | ��3 | ��1 | ��3 |
�ش��������⣺
��1���ĵ����ڿ����м��ȣ�������ĵ���ʽΪ_______________��
��2��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��___________��
��3���ȽϢٺ͢ߵ��⻯����ȶ��ԣ��û�ѧʽ��ʾ��_______________________________��
��4��д��ʵ�����Ʊ��ݵĵ��ʵ����ӷ���ʽ��________________________________��
��5��д����ĵ��ʸ��۵�����������ˮ�������Һ��Ӧ�����ӷ���ʽ��___________________________��
���𰸡�![]() HClO4 H2O>NH3 MnO2+4H++2Cl��
HClO4 H2O>NH3 MnO2+4H++2Cl��![]() Mn2++Cl2��+2H2O 2Al+2OH��+2H2O=2AlO2��+3H2��
Mn2++Cl2��+2H2O 2Al+2OH��+2H2O=2AlO2��+3H2��
��������
��û����ۡ�ֻ����ͼ�-2������֪��ΪO���ۢ������+1�����ڢ�A�壬�Ң۵�ԭ�Ӱ뾶�ϴ�ԭ�Ӱ뾶������С���ʢ�ΪLi����ΪNa���������+2�����ڢ�A�壬ԭ�Ӱ뾶����Li���ʢ�ΪMg���ܢ߶������+5����ͼ�-3�����ڢ�A�壬�Ңܵ�ԭ�Ӱ뾶�ϴʢ�ΪP����ΪN���������+7����ͼ�-1�����ΪCl����ֻ�����+3�����ڢ�A�壬ԭ�Ӱ뾶����P���ʢ�ΪAl���ݴ˽��
��û����ۡ�ֻ����ͼ�-2������֪��ΪO���ۢ������+1�����ڢ�A�壬�Ң۵�ԭ�Ӱ뾶�ϴ�ԭ�Ӱ뾶������С���ʢ�ΪLi����ΪNa���������+2�����ڢ�A�壬ԭ�Ӱ뾶����Li���ʢ�ΪMg���ܢ߶������+5����ͼ�-3�����ڢ�A�壬�Ңܵ�ԭ�Ӱ뾶�ϴʢ�ΪP����ΪN���������+7����ͼ�-1�����ΪCl����ֻ�����+3�����ڢ�A�壬ԭ�Ӱ뾶����P���ʢ�ΪAl��
(1)��ΪLiԪ�أ�����Li�ڿ����м���ֻ������Li2O�������ӻ���������ʽΪ![]() ��
��
(2)Ԫ�صķǽ�����Խǿ��������������ˮ���������Խǿ���ڢ�~~������Ԫ����������ۣ�����Ԫ������Ԫ�طǽ�������ǿ��������������ˮ�����У�������ǿ�Ļ�����ķ���ʽ��HClO4��
(3)��ΪOԪ�أ���ΪNԪ�أ��ǽ�����O����N�����⻯����ȶ���H2O>NH3��
(4)��Ϊ��Ԫ�أ�ʵ��������MnO2��Ũ�����ϼ�����������������Ӧ�����ӷ���ʽΪMnO2+4H++2Cl��![]() Mn2++Cl2��+2H2O��
Mn2++Cl2��+2H2O��
(5)��ΪAl����ΪNa��Al����NaOH��Һ����NaAlO2��H2�������ķ�Ӧ�����ӷ���ʽΪ2Al+2OH��+2H2O=2AlO2��+3H2����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�