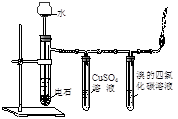��Ŀ����
ʵ�������Ҵ���Ũ���Ṳ����ȡ��ϩ�������¶ȹ��߶����������Ķ������������������ʵ����ȷ�������������������ϩ�Ͷ�������
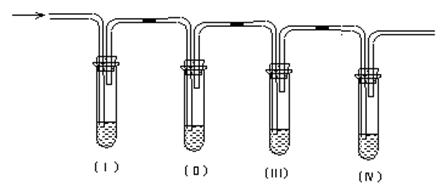
��1����װ�ÿ�ʢ�ŵ��Լ��ǣ�I ��II ��III ��IV ���������й��Լ����������ո��ڣ���
��2����˵����������������ڵ������� ______________________________________��
��3��ʹ��װ�â��Ŀ���� _________________________________________________ ��
��4��ʹ��װ�â��Ŀ����___________________________________________________��
��5��ȷ֤������ϩ��������_________________________________________________��

��1����װ�ÿ�ʢ�ŵ��Լ��ǣ�I ��II ��III ��IV ���������й��Լ����������ո��ڣ���
| A��Ʒ����Һ | B������������Һ | C��Ũ���� | D�����Ը��������Һ |
��3��ʹ��װ�â��Ŀ���� _________________________________________________ ��
��4��ʹ��װ�â��Ŀ����___________________________________________________��
��5��ȷ֤������ϩ��������_________________________________________________��
��1��ABAD; ��2�� ��1����Ʒ����ɫ; ��3����ȥ��������
��4��ȷ�϶�����������ȫ��������5�����Ը��������Һ��ɫ
��4��ȷ�϶�����������ȫ��������5�����Ը��������Һ��ɫ
�����������ϩ�Ͷ���������ʹ��ˮ��ɫ��Ҳ����ʹ���Ը��������Һ��ɫ�������ڼ�����ϩǰҪ�ȼ�������������ȥ��SO2��Ư���ԣ���ʹƷ����Һ��ɫ����Ʒ��������SO2�Ĵ��ڡ�SO2������������ܺͼ����Ӧ�����κ�ˮ��������������Һ��ȥ���������������Ƿ���ɾ�����Ʒ�������顣��������Ը��������Һ����֤��ϩ�Ĵ��ڡ�

��ϰ��ϵ�д�
�����Ŀ
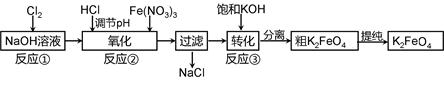
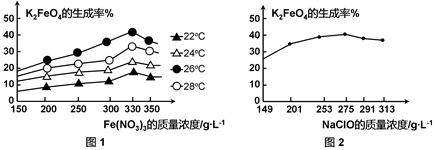
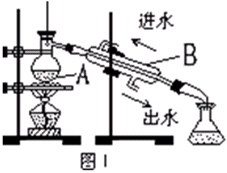
 4Fe(OH)3 + 8OH- + 3O2���ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���
4Fe(OH)3 + 8OH- + 3O2���ڡ��ᴿ��K2FeO4�в����ؽᾧ��ϴ�ӡ����º�ɵķ�������ϴ�Ӽ����ѡ�� ��Һ������ţ���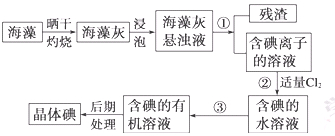

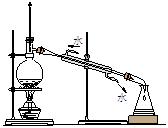
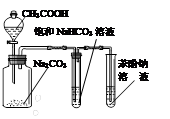 A��ʵ�����Ʊ���������ϩ
A��ʵ�����Ʊ���������ϩ