��Ŀ����
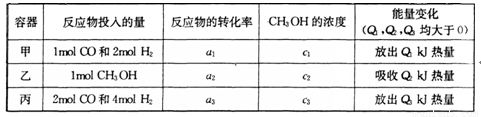
(15��)�״���ͨ����ú���������������ɵ�CO��H2��һ�������£��������·�Ӧ�Ƶã��������ͼʾ�ش��������⣺
(1) �ӷ�Ӧ��ʼ��ƽ�⣬��COŨ�ȱ仯��ʾƽ����Ӧ����v(CO)=________��
(2) д���÷�Ӧ���Ȼ�ѧ����ʽ________________
(3) ����������,���д�ʩ����ʹ�������________________
A�����¶� B����He��
C�ٳ���1molCO��2mol H2 Dʹ�ô���
(4) �����¶Ⱥ�������ͬ�������ܱ�������,����ͬ��ʽͶ�˷�Ӧ��.����÷�Ӧ�ﵽƽ�ⅼ���й��������±���
�����й�ϵ��ȷ����________
A c1=c2 B.
C. 2a1=a3 D. a1 +a2 =1
E�÷�Ӧ������1molCH3OH����ų�(Q1+Q2)kJ����
(5) ����һ����ɱ���ܱ������г���l mol CO 2mol H2��1mol CH3OH���ﵽƽ�ⅼ��û��������ܶ���ͬ��ͬѹ����ʼ��1.6������÷�Ӧ��________(����������桱)��Ӧ�����ƶ���������________________
1)0.075mol/L��min
(2)CO(g)+2H2(g)===CH3OH(g);��H=-91KJ/mol
(3) C (4) A D E
(5) �� ���÷�Ӧǰ����������������,ͬ��ͬѹ�´�ƽ��ʱ�����ܶ�����,�����������С,ƽ�������ƶ���
����:��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д���12�֣����ؼ״����ӽ���Ĥȼ�ϵ�أ�PEMFC)���״�����ת��Ϊ�����Ĺ� �������֣���1��ˮ�����任��������������2�����������������ֹ��ն��� ������ƷCO��
1���ֱ�д�������ֹ��յĻ�ѧ����ʽ��ͨ�����㣬˵�������ֹ��յ���ȱ�㡣�й����ϣ�298 .15K�����ڱ�3��
��3 ���ʵ�����ѧ����
���� | ��fHm | Sm |
CH3OH��g�� | -200.66 | 239.81 |
CO2��g�� | -393.51 | 213.64 |
CO��g�� | -110.52 | 197.91 |
H2O��g�� | -241.82 | 188.83 |
H2 ��g�� | 0 | 130.59 |
2���������ֹ��ղ���������CO��������ȼ�ϵ�ص�Pt������������������棬�谭H2�������͵�����������ȼ�ϵ�طŵ����ܼ����½���Ϊ�ˣ������˳�ȥCO�ķ���������һ��ʵ������500K�����4��

����PCO��PO2 �ֱ�ΪCO��O2�ķ�ѹ��rcoΪ��ÿ��ÿ������Ru����λ�������ĵ�CO��������ʾ��CO���������ʡ���1�������Ru��CO������Ӧ�ֱ��CO��O2�ķ�Ӧ������ȡ��������д�� ���ʷ��̡���2������Ru�����������������ӵ������������������ֻ�������ջ���λ�ſ��ܷ����������á������������ӵ����˶��Ķ������Կ˷�����������������ʱ�������Ѹ������»ص����ࡣ����CO��O2���������Ѹ�����Ӱ�죬���ұ����Ǿ��ȵģ��Ԧȱ�ʾ������Ӹ��ǻ���λ�İٷ��������Ƕȣ�������������������������ѹ�������ȣ�Ҳ��������Ŀջ���λ�������ȡ��о����CO��Ru�ϵ�������Ӧ��һ�ֻ������£�
 ����kco,ads�� kco,des�ֱ�ΪCO��Ru�Ļ���λ�ϵ��������ʳ������Ѹ����ʳ�����ko2,adsΪO2��Ru�Ļ���λ�ϵ��������ʳ�����M��ʾRu���������ϵĻ���λ��CO��Ru�������λ�ϵ�������O2������ǿ�öࡣ�Ը���������Ӧ�����Ƶ�CO�ڴ���Ru������������Ӧ�����ʷ��̣�������O2���Ѹ���Ҳ�����Dz���CO2��������������ʵ�����Ƚϡ�
����kco,ads�� kco,des�ֱ�ΪCO��Ru�Ļ���λ�ϵ��������ʳ������Ѹ����ʳ�����ko2,adsΪO2��Ru�Ļ���λ�ϵ��������ʳ�����M��ʾRu���������ϵĻ���λ��CO��Ru�������λ�ϵ�������O2������ǿ�öࡣ�Ը���������Ӧ�����Ƶ�CO�ڴ���Ru������������Ӧ�����ʷ��̣�������O2���Ѹ���Ҳ�����Dz���CO2��������������ʵ�����Ƚϡ�
3���й����ʵ�����ѧ������298��15 K�����5��
��5�����ʵ�����ѧ����
���� | ��fHm | Sm |
H2 ��g�� | 0 | 130.59 |
O2��g�� | 0 | 205.03 |
H2O ��g�� | -241.82 | 188.83 |
H2O ��l�� | -285.84 | 69.94 |
��373.15K��100kPa�£�ˮ�������ʦ�vap Hm![]() =40.64kJ?mol-1����298.15��3
=40.64kJ?mol-1����298.15��3
73.15K��ˮ�ĵ�ѹ����Ϊ75.6 J?K-1?mol-1����1�����������յõ��ĸ���������Ϊ���ӽ���Ĥȼ�ϵ�ص�ȼ�ϡ�ȼ�ϵ�ص�����Ч����ָ��������������繦�����ȼ�Ϸ�Ӧ�ʱ��Ч�ʡ���298.15K��100 kPa�£���1 molH2ȼ�շֱ�����H2O��l) �� H2O��g)ʱ������ȼ�ϵ�ع���������Ч�ʣ����������ߴ��ڲ���ԭ��2����ȼ�ϵ����473.15 K��100 kPa�¹�����������Ч����Ϊ���٣��ɺ����� ��͵ձ����¶ȵı仯������3��˵����1���ͣ�2���е�ͬһ��Ӧ�в�ͬ����Ч�ʵ�ԭ��
��ú����ת���ɺϳ�����Ȼ��ͨ��һ̼����·�ߺϳɸ�����Ʒ��ʯ����Ʒ��һ̼�����ļ�Ϊ��Ҫ�������й�����ǰ������δ���൱һ��ʱ�ڽ���Ϊһ̼��������Ҫ����ȥˮ�������ˮú����55��59%��H2��15��18%��CO��11��13%��CO2��������H2S��CH4����ȥH2S�ɲ��ô���Ǵ�ת����������CH4ת����CO���õ�CO��CO2��H2�Ļ�����壬������ĺϳɼ״�ԭ���������ɽ��м״��ϳɡ�
ˮú�����Ƽ״��������̿�ͼ����
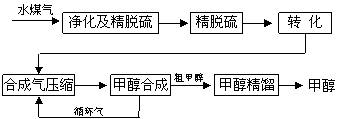 |
��1����ˮú������Ҫ��ѧ��Ӧ����ʽΪ��C��s��+H2O��g��![]() CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���� �˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ ��
CO��g��+H2��g�����˷�Ӧ�����ȷ�Ӧ���� �˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ ��
������������̼��ת���ʵĴ�ʩ�� ��(����ĸ����)
A������C��s�� B������H2O��g�� C�������¶� D������ѹǿ
��2���ϳ�����ѹ�����º����10m3�״��ϳ������ڴ��������£����м״��ϳɣ�
��Ҫ��Ӧ���£� 2H2(g) + CO(g) ![]() CH3OH(g)����H ����90.8kJ��mol��1��T4���´˷�Ӧ
CH3OH(g)����H ����90.8kJ��mol��1��T4���´˷�Ӧ
��ƽ�ⳣ��Ϊ160�����¶��£����ܱ������м���CO��H2����Ӧ��ijʱ�̲�ø���
�ֵ�Ũ�����£�
| ���� | H2 | CO | CH3OH |
| Ũ��/��mol��L��1�� | 0.2 | 0.1 | 0.4 |
�� �Ƚϴ�ʱ�����淴Ӧ���ʵĴ�С��v�� v�� ���>������<������)��
�� ������CO��H2����T5�淴Ӧ10min�ﵽƽ�⣬c(H2)��0.4 mol��L��1�����ʱ���ڷ�Ӧ����v(CH3OH) �� mol��1��(Lmin)��1��
��3�����������У��ϳ���Ҫ����ѭ������Ŀ���� ��
 �������ͼʾ�ش��������⣺
�������ͼʾ�ش��������⣺
 �������________________
�������________________

 �������ͼʾ�ش��������⣺
�������ͼʾ�ش��������⣺
 �������________________
�������________________