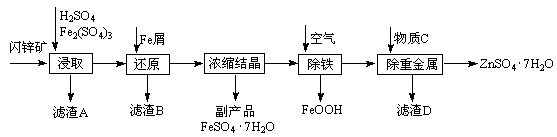
��Ŀ����
����ʵ���У�����ʵ����������ó��Ľ�����ȷ����
| | ���� | �֡��� | �ᡡ�� |
| A�� | �ⶨ��Ũ�ȵ�Na2CO3��Na2SO3 ��Һ��pH | ǰ��pH�Ⱥ��ߵĴ� | �ǽ����ԣ�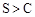 |
| B�� | ��ɫ��Һ�еμ���ˮ��CCl4�� ������ | �²���Һ����ɫ | ԭ��Һ����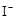 |
| C�� | ����ҺX�м���ϡ���ᣬ������������ɫ����ͨ�����ʯ��ˮ�� | ���ɰ�ɫ���� | ��ҺX��һ������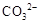 �� 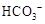 |
| D�� | ��ij��ɫ��Һ�еμ������ữ��BaCl2��Һ | ������ɫ���� | ��Һ��һ������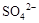 |
B
���������A����Ҫ�ȽϷǽ����ԣ�
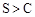 �����ԱȽ���������Ӧ��������̼���ǿ��������B����ȷ��C�в����ų���������ĸ��ţ�����D����ų���������ĸ��ţ�����
�����ԱȽ���������Ӧ��������̼���ǿ��������B����ȷ��C�в����ų���������ĸ��ţ�����D����ų���������ĸ��ţ�����
��ϰ��ϵ�д�
 �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
�����Ŀ
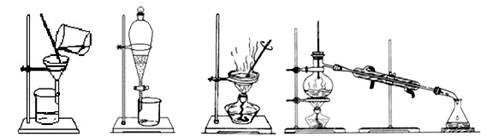
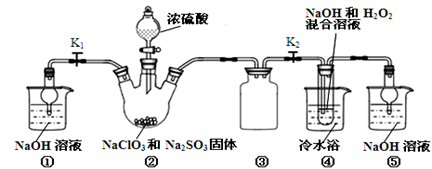
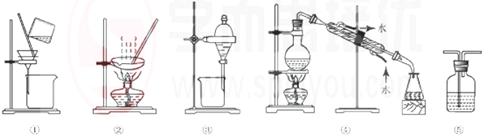
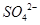 ��
��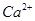 ��
��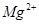 ��
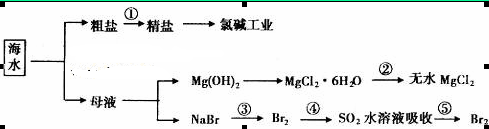
��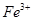 �����ʣ������ҩƷ˳��Ϊ��Na2CO3��Һ��NaOH��Һ��BaC12��Һ�����˺������
�����ʣ������ҩƷ˳��Ϊ��Na2CO3��Һ��NaOH��Һ��BaC12��Һ�����˺������ 6H2O���ڿ��������ȷֽ�����ˮMgC12
6H2O���ڿ��������ȷֽ�����ˮMgC12