��Ŀ����
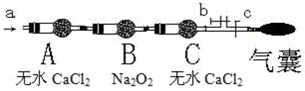
ij��һ��ѧ��ȤС��ͨ������ʵ����ȡ�����еĵ⣺�ٷ��麣��������һ����������������Һ�����̿��������ˮ���ݺ����ң����ˣ����������ữ��Һ����������(KIO3)������Һ�еĵ�����(I��)�����ɵⵥ�ʣ��������Ȼ�̼��ȡˮ��Һ�еĵ⣮��ش��������⣺
(1)ʵ�鲽��������õ�����Ҫ�����У�________(������)��
(2)ʵ�鲽�������ص����ӷ���ʽΪ��________________��
(3)ʵ�鲽�����Ҳ������H2O2����KIO3����صĻ�ѧ����ʽΪ��
________________________________��
(4)����ʵ�鲽��ܸ������������˵����ȷ���ǣ�________(��ѡ��)
A����Һǰ��Է�Һ©�����м�©
B��Ϊ��֤��Һ©���ڵ�Һ��˳�����������Խ��ϿڵIJ�������
C����Һʱ�����²�Һ��ӷ�Һ©���¶˷ų���һֻ�ձ��ٽ��ϲ�Һ��ӷ�Һ©���¶˷ų�
D�������Һ�����Һ©���в�����
E���ӷ�Һ©���Ͽڵ����ϲ�ˮ��Һ
�𰸣�
������
������
|
����(1)�ձ�����������©��������̨��(2��) ����(2) ����(3) ����(4)ABE��(2��) |

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ