��Ŀ����
18���л���Y����ȡ������֬���������͵���Ҫԭ�ϣ�PVAc��֬����������Ϳ����PVA���л���N���������϶���������㾫������������ǵĺϳ�·�����£�
��֪��RΪ������R'��R��Ϊ��������ԭ�ӣ�
��

��

�ش��������⣺
��1��A�ĵ���ʽ
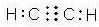
��2��D��������H��һ�������������ɸ���ˮ����֬I����д��H��I�Ļ�ѧ����ʽ��

��3��D�����������9��ԭ�ӹ���
��4��д��D��E�Ļ�ѧ����ʽ��
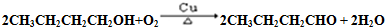
��5��д��M��N�Ļ�ѧ����ʽ��
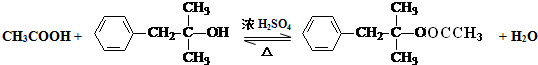
��6����B����ͬ�Ĺ����ŵ�ͬ���칹����4�֣�����˳���칹��
���� ����ϢI��Y�Ľṹ�����ƿ�֪GΪ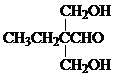
 ����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ
����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ ��MΪ
��MΪ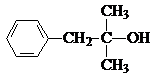
 ���ݴ˽��
���ݴ˽��
��� �⣺����ϢI��Y�Ľṹ�����ƿ�֪GΪ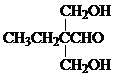
 ����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ
����N�ķ���ʽ��֪��������M����������Ӧ����N����M�ķ���ʽΪ��C12H16O2+H2O-C2H4O2=C10H14O����N�ķ����к���3��������M�к���2�����������ϢII��֪��KΪ ��MΪ
��MΪ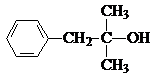
 ��
��
��1��AΪCH��CH��A�ĵ���ʽΪ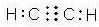 ��
��
�ʴ�Ϊ��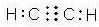 ��
��
��2��DΪCH3CH=CHCHO����HΪCH3CH=CHCOOH����һ��������CH3CH=CHCOOH�����Ӿ۷�Ӧ���ɸ���ˮ����֬I��H��I�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��3��DΪCH3CH=CHCHO������̼̼˫����̼��˫���ϵ�����ԭ�Ӷ����Թ��棬��������ת��������D�����������9��ԭ�ӹ��棬
�ʴ�Ϊ��9��
��4��D��E�Ļ�ѧ����ʽΪ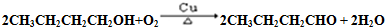 ��
��
�ʴ�Ϊ��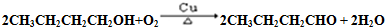 ��
��
��5��M��N�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6����B��CH3COOCH=CH2������ͬ�Ĺ����ŵ�ͬ���칹��ΪCH2=CHCOOCH3��HCOOCH=CHCH3����˳�������֣���HCOOCH2CH=CH2����4�֣�
�ʴ�Ϊ��4��
���� ���⿼���л����ƶϣ���Ҫѧ���Ը������Ϣ�������ã��ϺõĿ���ѧ����ѧ������Ǩ���������������������������������ת����ϵ���л���Ľṹ������ʽ�뷴Ӧ���������ƶϣ���Ҫѧ���������չ����ŵ�������ת�����Ѷ��еȣ�

| A�� | ԭ�Ӽ������ó�Ϊ��ѧ�� | |
| B�� | �ƻ��ɻ�ѧ������Ҫ�ų�һ�������� | |
| C�� | �ڻ�ѧ��Ӧ�в��������ʵı仯���������������ı仯 | |
| D�� | �������û�ѧ��Ӧ��ֻ��Ϊ����ȡ����Ҫ������ |
| A�� | ���ʵ���Ũ����ͬ��������Һ��PH��NaHCO3��Na2CO3 | |
| B�� | ��Ũ����ͬ��������Һ��Ӧ������������ʣ�NaHCO3��Na2CO3 | |
| C�� | ��ͬ�¶�ʱ��ˮ�е��ܽ��ԣ�NaHCO3��Na2CO3 | |
| D�� | ���ȶ��ԣ�NaHCO3��Na2CO3 |
| A�� | ���ö������̺�Ũ����������ʱ������������̺�Ӧ���ȵ�ȼ�ƾ��Ƽ��ȣ�Ȼ����μ���Ũ���� | |
| B�� | ʹ��ˮ���¶ȼƲ����ձ��е�ˮԡ�¶�ʱ����������ˮ�����õιܽ�ˮ����������ˮ���Сƿ�У����Ƶ��¶ȼƲ���װ����۵Ĺ��ƿ�� | |
| C�� | ���Թܼд��Թܵ��������ϼ�ס����ܿ�Լ$\frac{2}{3}$�����ֳ��Թܼг���ĩ�ˣ����м��� | |
| D�� | ������Һʱ��Һ��ɽ���Ӧ������ˮ��ȴ |
| A�� | 1 mol Fe������ϡ���ᷴӦʱ��ת�Ƶ��ӵ���ĿΪ3 NA | |
| B�� | ��״���£�22.4 Lˮ�к���ˮ���ӵ���ĿΪNA | |
| C�� | 14 g N2�к��е��ӵ���ĿΪ7 NA | |
| D�� | ��ĿΪNA��һ����̼���Ӻ�0.5 mol�����������Ϊ7��4 |
| A�� | ����ˮ�д�������ƽ�⣺Br2+H2O?HBr+HBrO��������NaOH��Һ����ɫ��dz | |
| B�� | ��2NO2��g��?N2O4��g�� ƽ����ϵ������ѹǿʹ��ɫ��dz | |
| C�� | ��ӦCO+NO2?CO2+NO��H��0�����¶�ʹƽ�����淽���ƶ� | |
| D�� | ��˫��ˮ��Һ�е���FeCl3 ��Һ�����������ʼӿ� |
| A�� | ${\;}_{8}^{16}$O2��${\;}_{8}^{18}$O2��Ϊͬλ�أ��������� | |
| B�� | ������ϡ�����У��ټ��������ƿ��Լӿ�ų����������� | |
| C�� | ������Ư�۳���������ˮ������ɱ����������������ԭ����ͬ | |
| D�� | C��ʯī��s��=C�����ʯ��s����H��0������ʯī�Ƚ��ʯ�ȶ� |