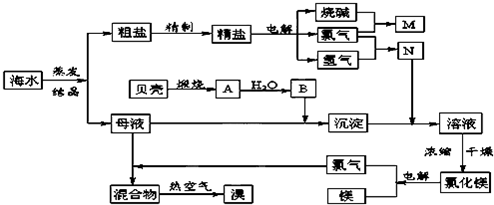
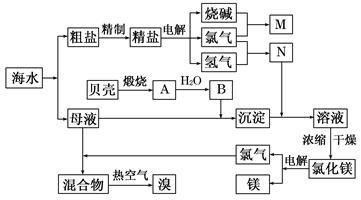
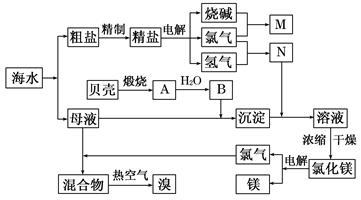
��Ŀ����
Ŀǰ��ҵ����Ҫ�������ӽ���Ĥ����ⱥ��ʳ��ˮ�����Ƶ�Cl2��H2�����Ի�õ��������������ᡣ����������Cl2��H2��1��a������ȣ���ͼʾ���̺ϳ���������Ϊ36.5%�����ᡣ![]()
![]()
ͼ2-3-4
(1)д����ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ________________________________________��
(2)��֪����������ʳ�ε��ܽ��Ϊ36.5 g������¶��±���ʳ��ˮ���ʵ���������Ϊ__________��
(3)��ÿ̨����ƽ��ÿ������2.3��104 molʳ�Σ�������ɵ������������������1��1.15ͨ��ϳ�¯�������Ǹ��ε�������ģ������Ͽ�����36.5%������__________t��
(4)������������1��a(a��1)ͨ��ϳ�¯����ó�ÿ������ʳ��c t�������Ǹ��ε�������ģ���ÿ������36.5%������__________b t��b=__________t��
������(2)���ڸ��¶���NaCl���ܽ��Ϊ36.5 g�����Ը��¶��±���NaCl����������Ϊ![]() ��100%=26.8%��
��100%=26.8%��
(3)�ɻ�ѧ����ʽ2NaCl+2H2O![]() 2NaOH+Cl2��+H2����H2+Cl2
2NaOH+Cl2��+H2����H2+Cl2![]() 2HCl��֪����ʹCl2��H2�������Ϊ1��1.15ͨ��ϳ����������1.15 mol H2����2.3 mol NaCl�ı�����Һ����ȫ��Ӧ�����2 mol HCl��������36.5%�����������Ϊx����
2HCl��֪����ʹCl2��H2�������Ϊ1��1.15ͨ��ϳ����������1.15 mol H2����2.3 mol NaCl�ı�����Һ����ȫ��Ӧ�����2 mol HCl��������36.5%�����������Ϊx����
2.3 NaCl �� 2HCl
2.3 mol 2��36.5 g
2.3��104 mol x��36.5%
x=![]()
(4) ��Cl2��������1��a(a��1)ͨ��ϳ�¯����(3)�еķ����ɵ����¹�ϵ��
aNaCl �� HCl
58.5a 36.5
c��106 g b��36.5%��106
��֮����![]() ��
��
�𰸣�(1)2NaCl+2H2O![]() 2NaOH+Cl2��+H2��
2NaOH+Cl2��+H2��
(2)26.8% (3)2.0 (4)![]()