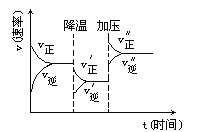
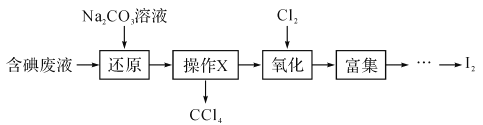
��Ŀ����
����Ŀ��X��Y��Z��W�����ֶ�����Ԫ�أ�Xԭ��M���ϵĵ�������ԭ�Ӻ�����Ӳ�����2����Y ԭ�������������Ǵ�����������2����ZԪ�صĵ���Ϊ˫ԭ�ӷ��ӣ�Z���⻯��ˮ��Һ�ʼ��ԣ�WԪ�����������+7�ۡ��ش��������⣺
(1) Ԫ��Xԭ�ӽṹʾ��ͼΪ_________________��
(2) Ԫ��Y��һ��ͬλ�ؿɲⶨ�������������ͬλ�صķ�����______________��
(3) Ԫ��W�ĵ�����Ԫ��X�ĵͼ���������ˮ��Һ�з�Ӧ�����ӷ���ʽΪ_________________________��
(4) ZW3����ˮ��Ӧ����һ�����һ�ּ��Ӧ�Ļ�ѧ����ʽΪ_________________________________��
���𰸡� ![]()
![]() C Cl2+SO2+2H2O=4H++2Cl-+ SO42- NCl3+4H2O=3HClO+NH3��H2O
C Cl2+SO2+2H2O=4H++2Cl-+ SO42- NCl3+4H2O=3HClO+NH3��H2O
��������Xԭ��M���ϵĵ�������ԭ�Ӻ�����Ӳ�����2����˵��X����3�����Ӳ㣬��M������6�����ӣ���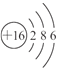 ��Ϊ��Ԫ�أ�Yԭ�������������Ǵ�����������2����˵��Yֻ��2�����Ӳ㣬���������4�����ӣ�YΪ̼Ԫ�أ�Z���⻯��ˮ��Һ�Լ��ԣ���ѧ�μ�������ֻ��NH3�����ZΪ��Ԫ�أ�W���������Ϊ+7����FԪ�������ۣ����WΪ��Ԫ�أ�
��Ϊ��Ԫ�أ�Yԭ�������������Ǵ�����������2����˵��Yֻ��2�����Ӳ㣬���������4�����ӣ�YΪ̼Ԫ�أ�Z���⻯��ˮ��Һ�Լ��ԣ���ѧ�μ�������ֻ��NH3�����ZΪ��Ԫ�أ�W���������Ϊ+7����FԪ�������ۣ����WΪ��Ԫ�أ�
(1)XΪ��Ԫ�أ�ԭ�ӽṹʾ��ͼΪ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
(2)̼�ķ�����ͬλ��146C�����ڿ��ţ��ʴ�Ϊ��146C��
(3)Cl2��SO2��ˮ��Һ�з�Ӧ���ӷ���ʽΪ��Cl2+SO2+2H2O�T4H++2Cl-+SO42-���ʴ�Ϊ��Cl2+SO2+2H2O�T4H++2Cl-+SO42-��
(4)NCl3��H2O��Ӧ���������мֻ����NH3H2O��NΪ-3�ۣ�ClΪ+1�ۣ���ӦΪHClO����Ӧ����ʽΪ��NCl3+4H2O�T3HClO+NH3H2O���ʴ�Ϊ��NCl3+4H2O�T3HClO+NH3H2O��

����Ŀ����һ��������ܱ������У��������»�ѧ��Ӧ��CO2��g��+H2��g��![]() CO��g����H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���£�
CO��g����H2O��g�����仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���£�
T/�� | 700 | 800 | 830 | 1000 | 1200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
��ش��������⣺
��1���÷�ӦΪ___________��Ӧ��������������������������
��2��830����c��CO��=0.01mol/L��c��H2O��=0.03mol/L��c��CO2��=0.01mol/L�� C��H2��=0.05mol/L���÷�Ӧ__________���������������������ﵽ�Ļ�ѧƽ��״̬��
��3��800��ʱ���̶��ݻ����ܱ������У����������ʼŨ��Ϊc��CO����0.01 mol/L��c��H2O����0.03 mol/L��c��CO2����0.01 mol/L��c��H2����0.05 mol/L����Ӧ��ʼʱ��H2O���������ʱ���������___________��������������С����������ȷ������
��4��830��ʱ����1L�Ĺ̶��ݻ����ܱ������з���2mol CO2��1mol H2��ƽ���CO2��ת����Ϊ___________��H2��ת����Ϊ___________���÷�����ʾ�������ٳ���1mol H2��H2��ת����Ϊ___________�������٣�������