��Ŀ����
��16�֣�ʵ������Ҫ230mL0.1mol/LNa2CO 3��Һ���ش���������
3��Һ���ش���������
(1) ������ҺʱӦѡ������ƿ�Ĺ��_______
(2) ����������ƽ������ʵ��Ӧ��̼���Ƶ�����_______
(3)���ѳƺõ�Na2 CO3����������Һ��������ƿ���Ҫ����������
CO3����������Һ��������ƿ���Ҫ����������
______ �� _ �� _____________
(4)����ʱ,����ȷ�IJ���˳��Ϊ (��ĸ��ʾ��ÿ����ĸֻ��һ��)
______ ______________
A.����������ˮ��ϴ 2��3 �Σ�����ϴҺ��������ƿ��[
B.���ƺõ�̼�����ƹ�������ձ��У�����������ˮ�����ܽ�
C.����ȴ���̼������Һת��������ƿ��
D.���Ǻ�ƿ�ǣ���ҡ��
E.���ý�ͷ�ι�С�ĵؼ�ˮ����Һ��Һ����̶������С�
F.������ƿ�ڼ�ˮ���̶��� 1��2 cm ��
(5)��ʵ�����������,��Һ��Ũ����ƫ��,ƫ�ͻ�����Ӱ��?
A����ˮʱԽ���̶��� _________
B������ʱ���ӿ̶��� __________
C������ƿ�ڱڸ���ˮ���δ���ﴦ�� ______
D�������ܽ��û����ȴ����ж��� _____
 3��Һ���ش���������
3��Һ���ش���������(1) ������ҺʱӦѡ������ƿ�Ĺ��_______
(2) ����������ƽ������ʵ��Ӧ��̼���Ƶ�����_______
(3)���ѳƺõ�Na2
 CO3����������Һ��������ƿ���Ҫ����������
CO3����������Һ��������ƿ���Ҫ����������______ �� _ �� _____________
(4)����ʱ,����ȷ�IJ���˳��Ϊ (��ĸ��ʾ��ÿ����ĸֻ��һ��)
______ ______________
A.����������ˮ��ϴ 2��3 �Σ�����ϴҺ��������ƿ��[
B.���ƺõ�̼�����ƹ�������ձ��У�����������ˮ�����ܽ�
C.����ȴ���̼������Һת��������ƿ��
D.���Ǻ�ƿ�ǣ���ҡ��
E.���ý�ͷ�ι�С�ĵؼ�ˮ����Һ��Һ����̶������С�
F.������ƿ�ڼ�ˮ���̶��� 1��2 cm ��
(5)��ʵ�����������,��Һ��Ũ����ƫ��,ƫ�ͻ�����Ӱ��?
A����ˮʱԽ���̶��� _________
B������ʱ���ӿ̶��� __________
C������ƿ�ڱڸ���ˮ���δ���ﴦ�� ______
D�������ܽ��û����ȴ����ж��� _____

��16�֣�
��1��250ml ��2�֣� ��2��2.7g ��3�֣�
��3���ձ�������������ͷ�ι� ��3�֣�
��4��BCAFED ��4�֣�
��5��A��ƫ�� B��ƫ�� C ����Ӱ�� D��ƫ�ߣ�ÿ��1�֣�
����Ӱ�� D��ƫ�ߣ�ÿ��1�֣�
��1��250ml ��2�֣� ��2��2.7g ��3�֣�
��3���ձ�������������ͷ�ι� ��3�֣�
��4��BCAFED ��4�֣�
��5��A��ƫ�� B��ƫ�� C
 ����Ӱ�� D��ƫ�ߣ�ÿ��1�֣�
����Ӱ�� D��ƫ�ߣ�ÿ��1�֣���

��ϰ��ϵ�д�
�����Ŀ
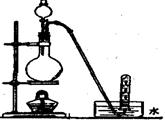
 ���ữ��BaCl2��Һ��ֻ�۲쵽�а�ɫ�������ɡ�
���ữ��BaCl2��Һ��ֻ�۲쵽�а�ɫ�������ɡ� ���������Ĺ�ϵ��ͼ��ʾ��
���������Ĺ�ϵ��ͼ��ʾ��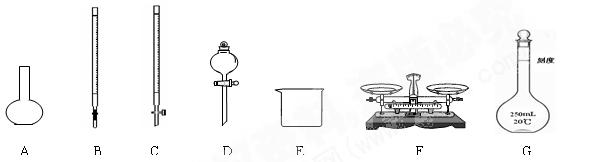 ���ʣ�
���ʣ�


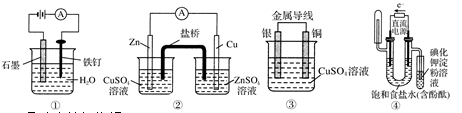
 ��
�� _____��
_____�� ����ʱ������������Һ
����ʱ������������Һ