��Ŀ����
��ҵ���������β���к��е�������NOx��NO��NO2�Ļ������費��N2O4��������̬���������ཡ�������ϴ����в��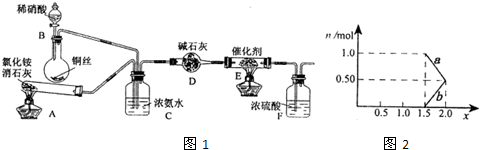
��1����ҵ�Ͽ��ð������շ�����NOx����Ӧԭ��Ϊ��4xNH3+6NOx
| ||
��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ
��װ��D�м�ʯ�ҵ�������
����ͬѧ��Ϊȥ��װ��AҲ���Դﵽʵ��Ŀ�ģ���������
��2����ҵ��Ҳ����Na2CO3��Һ���շ�����NOx����֪��NO������Na2CO3��Һ��Ӧ��
NO+NO2+Na2CO3�T2NaNO2+CO2��
2NO2+Na2CO3�TNaNO2+NaNO3+CO2��
�ٵ�NOx��Na2CO3��Һ��ȫ����ʱ��x��ֵ��������
A��1.3 B��1.6 C��1.8
�ڽ�1mol NOxͨ��Na2CO3��Һ�У�����ȫ����ʱ����Һ�����ɵ�NO3-��NO2-�������ӵ����ʵ�����x�仯��ϵ��ͼ2��ʾ��
ͼ���߶�a��ʾ
��������1�����Ȼ�粒�����������ƹ����ڼ��ȵ������·�����Ӧ���ɰ�����
�ڰ����Ǽ������壬�����ü�ʯ�������
��Ũ��ˮ���лӷ��ԣ����Բ���������
��2���ٵ�NOx��Na2CO3��Һ��ȫ����ʱ����n��NO2����n��NO�����ݴ����ش�
�����ü������غ㷨��������⼴�ɣ�
�ڰ����Ǽ������壬�����ü�ʯ�������
��Ũ��ˮ���лӷ��ԣ����Բ���������
��2���ٵ�NOx��Na2CO3��Һ��ȫ����ʱ����n��NO2����n��NO�����ݴ����ش�
�����ü������غ㷨��������⼴�ɣ�
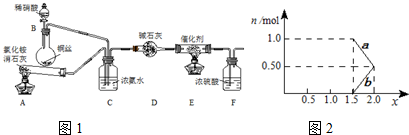
����⣺��1�����Ȼ�粒�����������ƹ����ڼ��ȵ������·�����Ӧ���ɰ���������װ��A�ķ�Ӧ�ǣ�2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
�ڰ����Ǽ������壬�����ü�ʯ�������װ��D�м�ʯ�ҵ������dz�ȥ�����е�ˮ������
�ʴ�Ϊ����ȥ�����е�ˮ������
��Cװ����Ũ��ˮ���лӷ��ԣ����Բ���������ȥ��װ��AҲ���Դﵽʵ��Ŀ�ģ�
�ʴ�Ϊ��Ũ��ˮ���лӷ��ԣ�NOx����װ��C�л����������
��2�����ɷ���ʽ��֪��NO�������ܱ����գ�NO��NO2������屻NaOH��Һ����ȫ���գ�����n��NO2����n��NO����1����n��NO2����n��NO��=1ʱxֵ��С��x��СֵΪ
=1.5����Ϊ����NO������x���ֵ��2����x��ȡֵ��ΧΪ1.5��x��2������x��ֵ��������1.3��
�ʴ�Ϊ��A��
���ü�������x=1.5����ӦΪNO��NO2�������ʵ�����Ϊ1��1������ʽ��Ӧ��û��NO3-����aӦ�ñ�ʾNO2-��
���غ㷨����Ӧ���ɵ�NaNO3��NaNO2�е�Ԫ������Ԫ��֮��Ϊ1��1������1mol NOx����ȫ����������̼����0.5mol������Ϊ53g�������̼������Һ������Ϊ250g��
�ʴ�Ϊ��NO2-��250��
| ||
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
| ||
�ڰ����Ǽ������壬�����ü�ʯ�������װ��D�м�ʯ�ҵ������dz�ȥ�����е�ˮ������
�ʴ�Ϊ����ȥ�����е�ˮ������
��Cװ����Ũ��ˮ���лӷ��ԣ����Բ���������ȥ��װ��AҲ���Դﵽʵ��Ŀ�ģ�
�ʴ�Ϊ��Ũ��ˮ���лӷ��ԣ�NOx����װ��C�л����������
��2�����ɷ���ʽ��֪��NO�������ܱ����գ�NO��NO2������屻NaOH��Һ����ȫ���գ�����n��NO2����n��NO����1����n��NO2����n��NO��=1ʱxֵ��С��x��СֵΪ
| 2+1 |
| 2 |
�ʴ�Ϊ��A��
���ü�������x=1.5����ӦΪNO��NO2�������ʵ�����Ϊ1��1������ʽ��Ӧ��û��NO3-����aӦ�ñ�ʾNO2-��
���غ㷨����Ӧ���ɵ�NaNO3��NaNO2�е�Ԫ������Ԫ��֮��Ϊ1��1������1mol NOx����ȫ����������̼����0.5mol������Ϊ53g�������̼������Һ������Ϊ250g��
�ʴ�Ϊ��NO2-��250��
���������⿼��ѧ������������������Լ��Ի�����Ӱ��֪ʶ��ע��֪ʶ�Ĺ��ɺ��������ѶȲ���

��ϰ��ϵ�д�
 ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ



 �£�
�£�
