��Ŀ����
ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ��������Բ�ͬ��һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ������ͼ����ʵ�����Ҫ�����������£�
������Ũ�Ⱦ�Ϊ1 mol��L-1������ʹ�����Һ��
����_________________��ȡ10.00 mL 1 mol��L-1����ʹ�����Һ�ֱ����������ƿ�У�
�۷ֱ��ȡ��ȥ��������Ĥ��þ��a g����ϵ��ͭ˿ĩ�ˣ�a����ֵ����Ϊ_____________��
���ڹ��ƿ��װ������ˮ������ͼ���Ӻ�װ�ã����װ�õ������ԣ�
�ݽ�ͭ˿�����ƶ���ʹ����þ����������(ͭ˿������Ӵ�)������Ӧ��ȫ����¼
__________________________________________________________________��
��Ӧ��������¶Ȼָ������£�������Һ���������Һ�棬��ȡ��Ͳ��ˮ�����ǰ��Ӧ______________________________________________��������Ͳ��ˮ�����ΪV mL��
�뽫�������貹������������������⣺
(1)�����ֱ����ܼ��װ�������ԵIJ�����۲췽����_______________________________��
(2)��ʵ����Ӧѡ��_________________ (�����)����Ͳ��
A.100 mL B.200 mL C.500 mL
(3)��ˮ������Ӱ����Բ��ƣ���ʵ���������£�����Ħ������ļ���ʽΪ��
Vm=_________________��
(4)�������ʲ��ȵ�ԭ����___________________________________________________��
ͭ˿������Ӵ���ԭ����___________________________________________________��
�������������о���ͬ��ͬѹ�£���ͬŨ�ȡ���ͬ��������Բ�ͬ��һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ������ļ���ʵ��װ�ã��ȼ��ڱ�����Һ������˵�������Ժã���ʵ���в����������Լ��112 mL������ѡ��200 mL����Ͳ��
�𰸣�
����ʽ�ζ��ܣ�
��0.12g;
�ݷ�Ӧ��ֹʱ�䡣
����Ͳ���������ƶ���ʹ�ҡ�����Һ����ȡ�
(1)�����ƽ�����ƿ���һ�ᣬ��۲쵽���ƿ���ҵ�������һ��ˮ��Һ������������װ�ò�©��
(2)B
(3)0.2V L��mol-1
(4)���Ũ����ͬʱ��������Ũ�Ȳ�ͬ����ֹ�γ�ԭ��أ�����ʵ������Ĺ۲�

 ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д� ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ����IJ�ͬǿ�ȵ�һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨ��ʵ�������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ����ʵ�����Ҫ�����������£�
ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ����IJ�ͬǿ�ȵ�һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨ��ʵ�������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ����ʵ�����Ҫ�����������£�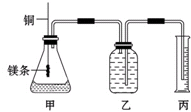 ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ����IJ�ͬǿ�ȵ�һԪ��������þ��Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ����ʵ�����Ҫ�����������£�
ij�о���ѧϰС��Ϊ֤����ͬ��ͬѹ�£���ͬŨ����ͬ����IJ�ͬǿ�ȵ�һԪ��������þ��Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ����ʵ�����Ҫ�����������£�
