��Ŀ����
��15�֣� �����ϡ��ķ��ֺ�ʹ�������Ἣ����ƶ�����������ķ�չ��һЩ���ϵij�������������̱�ʽ��ʱ�������塣��ش��������⣺
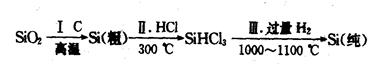
��1�����ǽ������ϡ��ߴ��ȵ������ǵ��͵����ǽ������ϣ��ֳơ��뵼�塱���ϣ����ķ��ֺ�ʹ��������������һ���������������ֲ��Ͽ������з����Ʊ���
��д������I�Ļ�ѧ����ʽ��_______________________________________��
�ڲ���II���������õ���SiHCl3���е�Ϊ33.0�棩�к���������SiCl4���е�Ϊ57.6�棩��HCl���е�Ϊ-84.7�棩���ᴿSiHCl3��ʵ�鷽����__________________________��
��2�����Բ��ϡ����ֲ���һ�㺬����Ԫ�أ�������;��Ϊ�㷺�Ľ�����Ҳ��Ϊ���ã����Դ�����ʹ�ÿ�ʼ�Ͳ���ʵʩ�Ŷ�����ʹ�ÿ�ʼ�Ͳ���ʵʩ�Ŷ����ķ��������������Ʒ��ʴ���ǵ绯ѧ��ʴ����д����Ϊ�ձ�ĵ绯ѧ��ʴ�ĸ�����Ӧʽ��_______________________��
��3��������ϡ��ҹ��Ǽ��⼼���Ƚ��Ĺ��ң��챦ʯ��Al2O3�����������ڲ�������IJ��ϣ�����һ������������������ӷ���ʽ����˵����______ _ ��______________��
��4���߷��Ӳ��ϡ������Էֳ����߷��Ӳ��Ϻ��л��߷��Ӳ��ϡ�һ������Ч��ˮ��[Al Fe (OH)n Cl6-n]m���������߷��Ӳ��ϣ����㷺Ӧ��������ˮ��ҵ��ˮ�Ĵ�����������Ԫ�صĻ��ϼ�Ϊ________��һ�ֺϳ���ά���ڣ��ֳơ�������ë�����ɱ�ϩ�棨CH2=CH-CN��Ϊԭ�Ͼۺ����ɣ���д�����ɸ��л��߷��Ӳ��ϵĻ�ѧ����ʽ�� _____________________��
��5���Ͻ���ϡ�14gͭ���Ͻ���һ����ijŨ�ȵ�������ȫ��Ӧ���ų���������1.12L����״���£�O2���ͨ��ˮ�У�ǡ��ȫ����ˮ���գ���Ͻ���ͭ������Ϊ_______g��
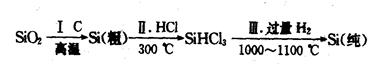
��1�����ǽ������ϡ��ߴ��ȵ������ǵ��͵����ǽ������ϣ��ֳơ��뵼�塱���ϣ����ķ��ֺ�ʹ��������������һ���������������ֲ��Ͽ������з����Ʊ���
��д������I�Ļ�ѧ����ʽ��_______________________________________��
�ڲ���II���������õ���SiHCl3���е�Ϊ33.0�棩�к���������SiCl4���е�Ϊ57.6�棩��HCl���е�Ϊ-84.7�棩���ᴿSiHCl3��ʵ�鷽����__________________________��
��2�����Բ��ϡ����ֲ���һ�㺬����Ԫ�أ�������;��Ϊ�㷺�Ľ�����Ҳ��Ϊ���ã����Դ�����ʹ�ÿ�ʼ�Ͳ���ʵʩ�Ŷ�����ʹ�ÿ�ʼ�Ͳ���ʵʩ�Ŷ����ķ��������������Ʒ��ʴ���ǵ绯ѧ��ʴ����д����Ϊ�ձ�ĵ绯ѧ��ʴ�ĸ�����Ӧʽ��_______________________��
��3��������ϡ��ҹ��Ǽ��⼼���Ƚ��Ĺ��ң��챦ʯ��Al2O3�����������ڲ�������IJ��ϣ�����һ������������������ӷ���ʽ����˵����______ _ ��______________��
��4���߷��Ӳ��ϡ������Էֳ����߷��Ӳ��Ϻ��л��߷��Ӳ��ϡ�һ������Ч��ˮ��[Al Fe (OH)n Cl6-n]m���������߷��Ӳ��ϣ����㷺Ӧ��������ˮ��ҵ��ˮ�Ĵ�����������Ԫ�صĻ��ϼ�Ϊ________��һ�ֺϳ���ά���ڣ��ֳơ�������ë�����ɱ�ϩ�棨CH2=CH-CN��Ϊԭ�Ͼۺ����ɣ���д�����ɸ��л��߷��Ӳ��ϵĻ�ѧ����ʽ�� _____________________��
��5���Ͻ���ϡ�14gͭ���Ͻ���һ����ijŨ�ȵ�������ȫ��Ӧ���ų���������1.12L����״���£�O2���ͨ��ˮ�У�ǡ��ȫ����ˮ���գ���Ͻ���ͭ������Ϊ_______g��
|
 Si��2CO�� ���������2��Fe��2e��=Fe2����3��Al2O3��6H��=2Al3����3H2O��Al2O3��2OH��=2AlO2����H2O
Si��2CO�� ���������2��Fe��2e��=Fe2����3��Al2O3��6H��=2Al3����3H2O��Al2O3��2OH��=2AlO2����H2O��4����3

��5��3.2g
��1���ٸ����£�̼�ܻ�ԭ�����������ɹ裬����ʽΪSiO2��2C Si��2CO����
Si��2CO����
������SiHCl3���е�Ϊ33.0�棩��SiCl4���е�Ϊ57.6�棩�е����Ƚϴ����Կ���ͨ����������ᴿ��
��2��������Ҫ�����绯ѧ��ʴ������������ʧȥ���ӣ�����ʽΪFe��2e��=Fe2����
��3��������������������������κ�ˮ���������ڼ������κ�ˮ�����Է���ʽΪAl2O3��6H��=2Al3����3H2O��Al2O3��2OH��=2AlO2����H2O��
��4�����ݻ�ѧʽ��֪�������ܼ��ǣ�6�������ǣ�3�۵ģ��������ǣ�3�۵ġ���ϩ���к���̼̼˫�����ܷ����Ӿ۷�Ӧ�����ɸ߷��ӻ��������ʽΪ ��
��
��5����Ӧ�в����������ǵ��������������Ӧ���ɵ������ᣬ���ԺϽ���ʧȥ�ĵ����൱�ڱ������õ�����ת�Ƶ�����1.12L��22.4L/mol��4��0.2mol���Ͻ���ͭ���������ʵ����ֱ���x��y����2x��y��0.2��64x��108y��14�����x��0.05mol��y��0.1mol������ͭ��������3.2g��
 Si��2CO����
Si��2CO����������SiHCl3���е�Ϊ33.0�棩��SiCl4���е�Ϊ57.6�棩�е����Ƚϴ����Կ���ͨ����������ᴿ��
��2��������Ҫ�����绯ѧ��ʴ������������ʧȥ���ӣ�����ʽΪFe��2e��=Fe2����
��3��������������������������κ�ˮ���������ڼ������κ�ˮ�����Է���ʽΪAl2O3��6H��=2Al3����3H2O��Al2O3��2OH��=2AlO2����H2O��
��4�����ݻ�ѧʽ��֪�������ܼ��ǣ�6�������ǣ�3�۵ģ��������ǣ�3�۵ġ���ϩ���к���̼̼˫�����ܷ����Ӿ۷�Ӧ�����ɸ߷��ӻ��������ʽΪ
 ��
����5����Ӧ�в����������ǵ��������������Ӧ���ɵ������ᣬ���ԺϽ���ʧȥ�ĵ����൱�ڱ������õ�����ת�Ƶ�����1.12L��22.4L/mol��4��0.2mol���Ͻ���ͭ���������ʵ����ֱ���x��y����2x��y��0.2��64x��108y��14�����x��0.05mol��y��0.1mol������ͭ��������3.2g��

��ϰ��ϵ�д�
�����Ŀ