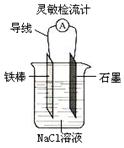
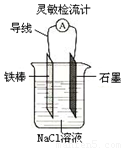
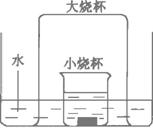
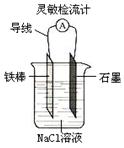
��Ŀ����
����ʵ��������ʵ����ʵ��������ȷ����
��ʵ���������Ȼ�������Һʱ�����Ȼ��������ܽ��������У�Ȼ��������ˮϡ�Ͳ������������ۡ�
������һ��Ũ�ȵ���Һʱ����������ƿ�Ŀ��ߣ���ʹ���Ƶ�Ũ��ƫ�ߣ�ʵ���Ҳⶨ�к���ʱ�����������ʹ�ⶨ���ƫ�͡�
�۽�Fe2(SO4)3��Һ�����������ɲ����գ����õ�����ɫ��ĩ��
��ʵ������ͭƬ��ϡ���ᷴӦ���������������ˮ���ռ���
���Թ��м����������ۣ��ټ���һ����ϡ���ᣬ����3��4���ӣ�Ȼ�����������Һ��Ƭ�̺�ܱ����С����������֡�
����ˮ�еμ�Al2(SO4)3��Һ����Al2(SO4)3��Һ�еμӰ�ˮ������ͬ��
�߱�����ˮ�����۵Ĵ��������Ʊ��屽��
��ֱ��������pH����ͬ������ʹ����еμӵ�Ũ�ȵ�����������Һ����ȫ�к�ʱ���ĵ�����������Һ�����һ���ࡣ
��ʵ���������Ȼ�������Һʱ�����Ȼ��������ܽ��������У�Ȼ��������ˮϡ�Ͳ������������ۡ�
������һ��Ũ�ȵ���Һʱ����������ƿ�Ŀ��ߣ���ʹ���Ƶ�Ũ��ƫ�ߣ�ʵ���Ҳⶨ�к���ʱ�����������ʹ�ⶨ���ƫ�͡�
�۽�Fe2(SO4)3��Һ�����������ɲ����գ����õ�����ɫ��ĩ��
��ʵ������ͭƬ��ϡ���ᷴӦ���������������ˮ���ռ���
���Թ��м����������ۣ��ټ���һ����ϡ���ᣬ����3��4���ӣ�Ȼ�����������Һ��Ƭ�̺�ܱ����С����������֡�
����ˮ�еμ�Al2(SO4)3��Һ����Al2(SO4)3��Һ�еμӰ�ˮ������ͬ��
�߱�����ˮ�����۵Ĵ��������Ʊ��屽��
��ֱ��������pH����ͬ������ʹ����еμӵ�Ũ�ȵ�����������Һ����ȫ�к�ʱ���ĵ�����������Һ�����һ���ࡣ
[ ]
A. �٢ڢܢ�
B. �٢ۢܢݢ�
C. �ۢܢޢ�
D. �ۢߢ�
B. �٢ۢܢݢ�
C. �ۢܢޢ�
D. �ۢߢ�
A

��ϰ��ϵ�д�
�����Ŀ