��Ŀ����
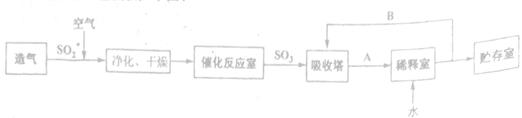
����ͼ��ʾװʵ���ý���ͭ��Ũ���ᷴӦ��ʵ��̽������ش��������⣺
��1���Թ�A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2���Թ�B�е�ʵ�������� �����Թ�B�з�Ӧ�����Һ���м��ȣ��۲쵽�Թ�B�е�ʵ�������� ��
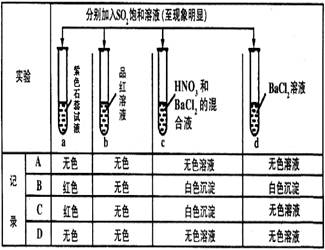
��3����Ӧһ��ʱ����Թ�C��û�й۲쵽�������ɣ������Թ�C��ͨ������һ������X�������ɰ�ɫ������������X�����������е� ��������ţ�
a��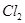 b��
b��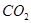 c��
c��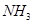 d��
d��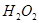
��4���Թ�A�е�ͭ�������ַ�Ӧ��ͭ�����ᶼ��ʣ�࣬������������ʹ�Թ�A�е�ͭƬ�����ܽ���� ��������ţ�
a��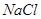 b��
b��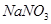 c��
c��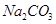 d��
d��
��5���Թ�D�е��Լ��� ��Һ���������� ��

��1���Թ�A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2���Թ�B�е�ʵ�������� �����Թ�B�з�Ӧ�����Һ���м��ȣ��۲쵽�Թ�B�е�ʵ�������� ��
��3����Ӧһ��ʱ����Թ�C��û�й۲쵽�������ɣ������Թ�C��ͨ������һ������X�������ɰ�ɫ������������X�����������е� ��������ţ�
a��
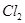 b��
b��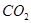 c��
c��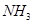 d��
d��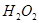
��4���Թ�A�е�ͭ�������ַ�Ӧ��ͭ�����ᶼ��ʣ�࣬������������ʹ�Թ�A�е�ͭƬ�����ܽ���� ��������ţ�
a��
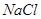 b��
b��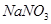 c��
c��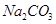 d��
d��
��5���Թ�D�е��Լ��� ��Һ���������� ��
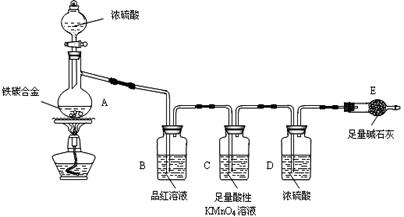
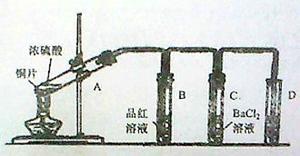
��13�֣���1��Cu��2H2SO4(Ũ) CuSO4��2H2O��SO2����3�֣�
CuSO4��2H2O��SO2����3�֣�
��2��Ʒ����Һ��ɫ��Ʒ����Һ�ָֻ�Ϊԭ������ɫ
��3�� c ����4�� b ����5�� NaOH��Һ�����ն�����������
 CuSO4��2H2O��SO2����3�֣�
CuSO4��2H2O��SO2����3�֣���2��Ʒ����Һ��ɫ��Ʒ����Һ�ָֻ�Ϊԭ������ɫ
��3�� c ����4�� b ����5�� NaOH��Һ�����ն�����������
�����������1��Ũ�������ǿ�����ԣ�����������ͭ�����Թ�A�з�����Ӧ�Ļ�ѧ����ʽΪCu��2H2SO4(Ũ)
 CuSO4��2H2O��SO2����
CuSO4��2H2O��SO2������2��SO2����Ư���ԣ����Թ�B�е�ʵ��������Ʒ����Һ��ɫ������SO2��Ư�����Dz��ȶ��ģ����ڼ��ȵ��������ָֻ���ԭ������ɫ�����Թ�B�з�Ӧ�����Һ���м��ȣ��۲쵽�Թ�B�е�ʵ��������Ʒ����Һ�ָֻ�Ϊԭ������ɫ��
��3��SO2����ˮ���������ᣬ��Һ�����ԣ��ò��������ᱵ��ɫ���������Ҫ���ɰ�ɫ��������Լ�����������������壬��ѡ��abd�����ԣ�����ad���ɵ������ᱵ��ɫ������c�ǵ������ᱵ��ɫ��������ѡb��
��4�������������£������ξ���ǿ�����ԣ�����������ͭ����˼���������������ƣ���ѡb��
��5��SO2�Ǵ�����Ⱦ�Ӧ��������������Һ���գ���ֹ��Ⱦ������
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣���������߿���������ǿ����ע�ض�ѧ������֪ʶ���̺�ѵ����ͬʱ�����ض�ѧ������������������������ѧ���������������淶�Ͻ���ʵ�����������Ҳ�����ڵ���ѧ����ѧϰ��Ȥ��ѧϰ�����ԡ�

��ϰ��ϵ�д�
�����Ŀ