��Ŀ����
Na2O2��ˮ�ķ�Ӧʵ����Na2O2+2H2O=2NaOH+H2O2����Ӧ���ȣ���Ӧ�ų�������ʹ����H2O2���ȷֽ⣺2H2O2=2H2O+O2����Ϊ�˲ⶨij�������ƹ���Ĵ��ȣ�������ʵ�飺

�ٳ�ȡ�������ƹ���2.00g��
�ڰ���Щ�������ƹ���������������õ����巢��װ���У�
������������еμ�ˮ����ijһ��Ͳ��ˮ��������Ͳ��Һ����112mL��ǡ����ˮ����Һ����ƽ��
�ܽ���ƿ�е�Һ��ת�Ƶ�250mL������ƿ�У�Ȼ���������ˮ�����ݣ�ʹҺ��ǡ����̶�����
������Һ����ȡ25.00mL����ƿ�е�Һ�壬������ƿ�У��ù�����ϡ�����ữ��Ȼ����0.0100mol/L��KMnO4��Һȥ�ζ������յ�ʱ��ȥ��24.20mLMnO4��Һ����ʱ��ȫ����Mn2+���ڣ�
��1������ʽ�ͼ�ʽ���ֵζ��ܱ��ã���ʵ��Ӧѡ�� �������� ��
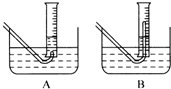
��2���ڲ���۲����������ʱ��������Թܺ���Ͳ�ڵ����嶼��ȴ������ʱ���У�Ӧѡ����ͼװ���� ��Ϊʹ��ͲΪҺ��ǡ����ˮ����Һ����ƽ��Ӧ���еIJ����� ��
��3��������KMnO4��Һ�ζ������Ƶ���Һ��������Ӧ�����ӷ���ʽ�� ��
��4���ڲ�����еζ��ﵽ�յ�ʱ����Һ����ɫ�仯�� ��
��5���ù������ƵĴ���Ϊ ��ʵ���еõ��������������Ϊ��״���£���
��1����ʽ�ζ��ܣ����Ը��������ǿ�����ԣ��ḯʴ��ʽ�ζ��ܵĽ��ܣ����Բ����ü�ʽ�ζ���ʢװ���������Һ��
��2��A ����Ͳ��ֱ�����ƣ�ֱ����Ͳ����Һ����ƽ��
��3��2MnO4�D+5H2O2=2Mn2++8H2O+5O2��
��4����Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
��5��62.6%

| A��Na2O�����ں������ | B��Na2O���ȶ����ܼ�����O2��������Na2O2 | C��Na2O2�ǰ�ɫ���壬����ˮ���õõ�O2��NaOH | D��Na2O2��ˮ�ķ�Ӧ�У���������Na2O2����ԭ����ˮ |
| A��Na2O�����ں������ | B��Na2O���ȶ����ܼ�����O2��������Na2O2 | C��Na2O2�ǰ�ɫ���壬����ˮ���õõ�O2��NaOH | D��Na2O2��ˮ�ķ�Ӧ�Ǹ��ֽⷴӦ |