��Ŀ����
���ձ���ʢ�а뱭��Ba��OH��2��Һ��Ȼ���õι����ձ��еμ�H2SO4��װ������ͼ������������ĵ��룬��ƻ��䰵��������ǡ����ȫ��Ӧʱ�������ȫϨ��
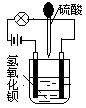
��1����ʵ��˵����Һ�ĵ�������_________________�йء�
��2��������Ӧ�����ӷ���ʽΪ_________________________________________��
��3��������������ԭ��������ҺŨ�ȵIJⶨ�����ձ���װ��75mL 0��1mol/L Ba��OH��2��Һ��������25mL H2SO4ʱ�������ȫϨ�������������Һ�����ʵ���Ũ�ȡ�
��4������98%��1��84g/mL��Ũ������������Ũ�ȵ�������Һ2500mL����Ҫ���ٺ�����Ũ���ᣨ��ȷ��0��1mL)��

��1����ʵ��˵����Һ�ĵ�������_________________�йء�
��2��������Ӧ�����ӷ���ʽΪ_________________________________________��
��3��������������ԭ��������ҺŨ�ȵIJⶨ�����ձ���װ��75mL 0��1mol/L Ba��OH��2��Һ��������25mL H2SO4ʱ�������ȫϨ�������������Һ�����ʵ���Ũ�ȡ�
��4������98%��1��84g/mL��Ũ������������Ũ�ȵ�������Һ2500mL����Ҫ���ٺ�����Ũ���ᣨ��ȷ��0��1mL)��
��1������Ũ�ȣ�2�֣�
��2��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O��3�֣�
��3��0��3mol/L��5�֣�
��4��40��8mL��4�֣�
��2��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O��3�֣�
��3��0��3mol/L��5�֣�
��4��40��8mL��4�֣�
�����������ϡ������Һ�������μ�����������Һֱ�����������ڷ����˷�Ӧ��Ba(OH)2 +H2SO4�TBaSO4��+2H2O����������������Һ�ĵμӣ�����Ũ����С�����������ͣ��������Ϊ�㣬Ҳ����˵ǡ����ȫ��Ӧʱû�������ƶ��������ˣ��������ʹ��������ܹ������ˣ���Һ����Һ�ĵ����Ա仯��ǿ������ˮ�����ĵ����ԣ���ǿ��
�Ÿ�ʵ��˵����Һ�ĵ�����������Ũ���йأ��𰸣�����Ũ�ȣ���������Ӧ�����ӷ���ʽΪ��Ba2++2OH-+2H++SO42-==BaSO4��+2H2O���𰸣�Ba2++2OH-+2H++SO42-==BaSO4��+2H2O���ǵ����ȫϨ��˵����Ӧ�պ���ȫ���У��ɷ���ʽ��֪��
Ba(OH)2 + H2SO4�TBaSO4��+2H2O
1 1
75mL��0��1mol/L C��25mL
75mL��0��1mol/L=C��25mL
C=0��3mol/L
�𰸣�0��3mol/L��
������98%��1��84g/L��Ũ������������Ũ�ȵ�������Һ2500mL��ŨH2SO4�����ʵ���Ũ��c=
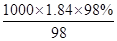 mol��L��1=18��4mol��L��1������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18��4mol��L��1=2500mL��0��3mol��L��1����ã�x��40��8������Ӧ��ȡ��Ũ���������40��8mL���𰸣�40��8mL��
mol��L��1=18��4mol��L��1������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18��4mol��L��1=2500mL��0��3mol��L��1����ã�x��40��8������Ӧ��ȡ��Ũ���������40��8mL���𰸣�40��8mL��
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ