��Ŀ����
��8�֣�ʵ�����мס�����ƿ��ʧ��ǩ����ɫ��Һ������һƿ�����ᣬ��һƿ��Na2CO3��Һ��Ϊȷ���ס��ҵijɷּ������ʵ���Ũ�ȣ��ֲ������£�
��ȡ25.00 mL����Һ�������л����μ�����Һ15.00mL�����ռ�������224mL��
����ȡ15.00mL����Һ�������л����μӼ���Һ25.00mL�����ռ�������112mL��
��������������ѻ���Ϊ��״����CO2����Һ�е������ܽ���Բ��ơ������ʵ��������գ�
��1������ ������Һ�����ʵ����ʵ���Ũ��Ϊ ������Һ�����ʵ����ʵ���Ũ��Ϊ ��
������Һ�����ʵ����ʵ���Ũ��Ϊ ������Һ�����ʵ����ʵ���Ũ��Ϊ ��
��2����n mL�ļ���Һ������������Һ�����ֿ��ܵķ�ʽ��ϣ��������������Ϊ
V mL(��״��)����V��ȡֵ��ΧΪ ��
��ȡ25.00 mL����Һ�������л����μ�����Һ15.00mL�����ռ�������224mL��
����ȡ15.00mL����Һ�������л����μӼ���Һ25.00mL�����ռ�������112mL��
��������������ѻ���Ϊ��״����CO2����Һ�е������ܽ���Բ��ơ������ʵ��������գ�
��1������
 ������Һ�����ʵ����ʵ���Ũ��Ϊ ������Һ�����ʵ����ʵ���Ũ��Ϊ ��
������Һ�����ʵ����ʵ���Ũ��Ϊ ������Һ�����ʵ����ʵ���Ũ��Ϊ ����2����n mL�ļ���Һ������������Һ�����ֿ��ܵķ�ʽ��ϣ��������������Ϊ
V mL(��״��)����V��ȡֵ��ΧΪ ��
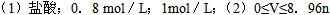
��

��ϰ��ϵ�д�
�����Ŀ
 4.7��10��8��д����84����Һ��(��Ҫ�ɷ�Ϊ�Ȼ��ƺʹ�������)��ͨ�������̼������Ӧ�����ӷ���ʽ ��
4.7��10��8��д����84����Һ��(��Ҫ�ɷ�Ϊ�Ȼ��ƺʹ�������)��ͨ�������̼������Ӧ�����ӷ���ʽ ��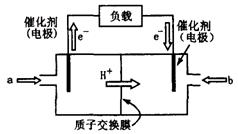
 ��ͼ������ܷ�ӦΪ2CH3OH��3O2
��ͼ������ܷ�ӦΪ2CH3OH��3O2 2CO2��4H2O����װ�÷ŵ�ʱ ���a����b����Ϊ��صĸ�������缫��ӦʽΪ ��
2CO2��4H2O����װ�÷ŵ�ʱ ���a����b����Ϊ��صĸ�������缫��ӦʽΪ ��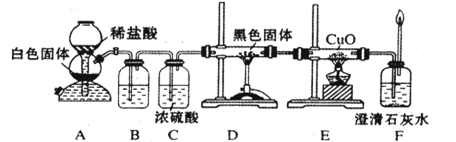
 CO+H2 CO + H2O
CO+H2 CO + H2O H2 C + CO2
H2 C + CO2 2CO
2CO 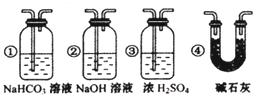
 Si���ֹ裩
Si���ֹ裩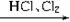 SiHCl3���е�31��5�棩
SiHCl3���е�31��5�棩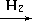 Si
Si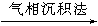 �����裨�ྦྷ�裩
�����裨�ྦྷ�裩 ������
������ ��������������
��������������