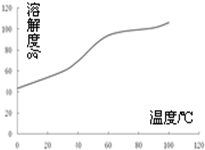
��Ŀ����
19������������Ԫ��W��X��Y��Zԭ��������������Wԭ�ӵ�������������Xԭ����������������������������Xԭ����5����Yԭ�ӵ������������Ǵ�����������һ�룬Z��W��ͬһ���壮����˵����ȷ���ǣ�������| A�� | ԭ�Ӱ뾶��W��X��Y��Z | |
| B�� | X��Y��Z���ʵ��۵㣺Z��Y��X | |
| C�� | ����̬�⻯������ȶ��ԣ�Y��Z��W | |
| D�� | ZԪ�ص�����������Ӧˮ����ķ����д��ڷǼ��Թ��ۼ� |
���� Wԭ�ӵ�������������Xԭ����������������������������X��5����ӦΪ�ڶ�����Ԫ�أ�XΪ��������Ԫ�أ���W������������Ϊ2x����X������������Ϊy�����У�2+8+x��-5=2+2x��x=3����W��ԭ������Ϊ8��ΪOԪ�أ�X��ԭ��Ϊ13��ΪAlԪ�أ�Z��W��ͬһ���壬��ZΪSԪ�أ�Yԭ�ӵ������������Ǵ�����������һ�룬��ΪC��SiԪ�أ�����Y��ԭ����������O������YΪSi���Դ˽����⣮
��� �⣺Wԭ�ӵ�������������Xԭ����������������������������X��5����ӦΪ�ڶ�����Ԫ�أ�XΪ��������Ԫ�أ���W������������Ϊ2x����X������������Ϊy�����У�2+8+x��-5=2+2x��x=3����W��ԭ������Ϊ8��ΪOԪ�أ�X��ԭ��Ϊ13��ΪAlԪ�أ�Z��W��ͬһ���壬��ZΪSԪ�أ�Yԭ�ӵ������������Ǵ�����������һ�룬��ΪC��SiԪ�أ�����Y��ԭ����������O������YΪSi������
A�����Ӳ���Խ�࣬ԭ�Ӱ뾶Խ���Ӳ���ͬʱ��ԭ������Խ��뾶ԽС����ԭ�Ӱ뾶��W��Z��Y��X����A����
B��XΪAl��YΪSi��ZΪS���䵥�ʵ��۵㣺S��Al��Si����B����
C��Ԫ�صķǽ�����Խǿ�����⻯��Խ�ȶ��������̬�⻯������ȶ��ԣ�Y��Z��W����C��ȷ��
D��ZΪS��SԪ�ص�����������Ӧˮ����Ϊ���ᣬ�������ֻ�м��Լ��������ڷǼ��Թ��ۼ�����D����
��ѡC��
���� ���⿼��Ԫ�ص��ƶϣ���Ŀ�Ѷ��еȣ���ȷ�ƶ�Ԫ�ص�����Ϊ������Ĺؼ���ע��������Ԫ�ػ���������ʣ�

 �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�2Na2O2+2SO3�T2Na2SO4+O2�ݴˣ�����Ϊ���з�Ӧ����ʽ����ȷ���ǣ�������
| A�� | 2Na2O2+2N2O4�T4NaNO3 | B�� | Na2O2+2NO2�T2NaNO2+O2 | ||
| C�� | 2Na2O2+2N2O3�T4NaNO2+O2 | D�� | 2Na2O2+2 Mn2O7�T4Na2MnO4+O2 |
| A�� | A��Ca��ClO��2��aq��$\stackrel{CO_{2}}{��}$HClO��aq��$\stackrel{����}{��}$HCl��aq�� | |
| B�� | NH3$��_{������}^{O_{2}}$NO$\stackrel{O_{2}}{��}$NO2$\stackrel{H_{2}O}{��}$HNO3 | |
| C�� | Fe$��_{��}^{����Cl_{2}}$FeCl2$\stackrel{NaOH��Һ}{��}$Fe��OH��2$\stackrel{�����з���}{��}$Fe��OH��3 | |
| D�� | H2SiO3$\stackrel{��}{��}$SiO2$\stackrel{NaOH��aq��}{��}$Na2SiO3 |
��ش��������⣺
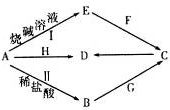
��1��д��F�ĵ���ʽ
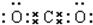 ��ʵ������ȡG�Ļ�ѧ����ʽΪCa��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2H2O+2NH3����
��ʵ������ȡG�Ļ�ѧ����ʽΪCa��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2H2O+2NH3������2��д����ӦI�����ӷ���ʽ2Al+2OH-+2H2O=2AlO2-+3H2�����÷�Ӧ�е���������H2O��
��3����ӦI������������ԴH2��������֪H2��ȼ����Ϊ286kJ•mol-1��18gˮ�������Һ̬ˮ�ų�44kJ����������������������±���
| O=O��g�� | H-H��g�� | H-O��g�� | |
| ����/kJ•mol-1 | 496 | x | 463 |
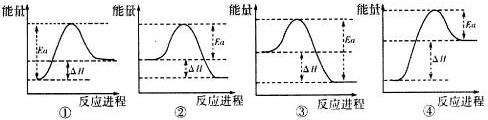
��4��д��A��H��Ӧ�Ļ�ѧ����ʽ3Fe3O4+8Al$\frac{\underline{\;����\;}}{\;}$9Fe+4Al2O3��ʵ���ø÷�Ӧ���ʱ䣨��H���ͻ�ܣ�Ea��������������ϵͼ�������Ǣڢۣ���д��ţ���
��5��������ʾH�����ڳ�ȥ����ˮ�е�TcO4-��99Tc���з����ԣ��������������£�H��TcO4-ת��Ϊ������ˮ��TcO2��ͬʱ�õ�һ��������ˮ������÷�Ӧ�����ӷ���ʽΪ3Fe3O4+TcO4-+13H2O+H+=TcO2+9Fe��OH��3��
| A�� | HCN�Ľṹʽ��H-C��N | |
| B�� | S2-�Ľṹʾ��ͼ��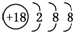 | |
| C�� | HClO�ĵ���ʽ��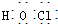 | |
| D�� | ������Ϊ86��������Ϊ51���ԭ�ӣ�$\stackrel{137}{86}$Cs |
| A�� | �Ȼ�����Һ�м��������ˮ��Ӧ��ʵ����Al3++3NH3•H2O�TAl��OH��3��+3NH${\;}_{4}^{+}$ | |
| B�� | ��������ˮ�е��ؽ������ӣ�����Ͷ�������ȵ���ʵķ������д��� | |
| C�� | þ���Ͻ�ȿ���ȫ���ڹ��������ֿ���ȫ���ڹ���NaOH��Һ | |
| D�� | �������ȷ�Ӧԭ�����ܷ�����Ӧ2Al+3MgO$\frac{\underline{\;����\;}}{\;}$3Mg+Al2O3 |
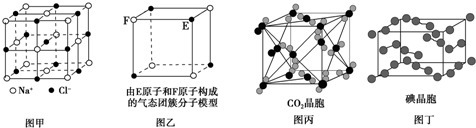
| A�� | ��NaCl���壨ͼ�ף��У���Na+�����Cl-�γ��������� | |
| B�� | ����̬�Ŵط��ӣ�ͼ�ң��ķ���ʽΪE4F4��F4E4 | |
| C�� | ��CO2���壨ͼ�����У�һ��CO2������Χ��12��CO2���ӽ��� | |
| D�� | �ڵ⾧�壨ͼ�����У�����ӵ�����ֻ��һ�ַ��� |