��Ŀ����
ʵ�������ܶ�Ϊ1.84g/cm3�����ʵ���������Ϊ98%�����ᣬ����200mL���ʵ���Ũ��Ϊ0.46mol/L�����ᣮ
��1��Ӧ��ȡ98%����������Ϊ______mL���������������õ��������������÷ֱ���______��______��
��2��ʹ������ƿǰ������еĵ�һ��������______��
��3��������Һʱ��һ����Է�Ϊ���¼������裺
�ٳ��� �ڼ��� ���ܽ� ��ҡ�� ��ת�� ��ϴ�� �߶��� ����ȴ
����ȷ�IJ���˳��Ϊ______��
�⣺��1��Ũ��������ʵ���Ũ��Ϊc=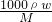 =
=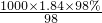 mol/L=18.4mol/L��
mol/L=18.4mol/L��
������Ũ��������ΪV������V��18.4mol/L=O.2L��0.46mol/L����V=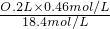 =O.005L=5mL��
=O.005L=5mL��
���������ܽ�ʱ�������ã�����Һʱ���������ã�
�ʴ�Ϊ��5�����裻������
��2������ƿ��ʹ��ǰ����Ƿ�©ˮ���ʴ�Ϊ������Ƿ�©ˮ��
��3�����������г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��������1���ȼ����Ũ��������ʵ���Ũ��Ϊc=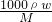 ��Ȼ�������Һϡ��ǰ�����ʵ��������������Ũ�������������ݲ�������ʵ���е�Ӧ�ã����ݲ�������ʵ���е����ã�
��Ȼ�������Һϡ��ǰ�����ʵ��������������Ũ�������������ݲ�������ʵ���е�Ӧ�ã����ݲ�������ʵ���е����ã�
��2������ƿǰʹ��ǰ����Ƿ�©ˮ��
��3������Ũ��Һ������ϡ��Һ��ʵ��������������
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע��ʵ��Ļ�������������ע�����
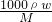 =
=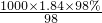 mol/L=18.4mol/L��
mol/L=18.4mol/L��������Ũ��������ΪV������V��18.4mol/L=O.2L��0.46mol/L����V=
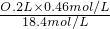 =O.005L=5mL��
=O.005L=5mL�����������ܽ�ʱ�������ã�����Һʱ���������ã�
�ʴ�Ϊ��5�����裻������
��2������ƿ��ʹ��ǰ����Ƿ�©ˮ���ʴ�Ϊ������Ƿ�©ˮ��
��3�����������г������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������ʴ�Ϊ���ڢ٢ۢ�ݢޢߢܣ�
��������1���ȼ����Ũ��������ʵ���Ũ��Ϊc=
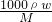 ��Ȼ�������Һϡ��ǰ�����ʵ��������������Ũ�������������ݲ�������ʵ���е�Ӧ�ã����ݲ�������ʵ���е����ã�
��Ȼ�������Һϡ��ǰ�����ʵ��������������Ũ�������������ݲ�������ʵ���е�Ӧ�ã����ݲ�������ʵ���е����ã���2������ƿǰʹ��ǰ����Ƿ�©ˮ��
��3������Ũ��Һ������ϡ��Һ��ʵ��������������
���������⿼����һ�����ʵ���Ũ����Һ�����ƣ��ѶȲ���ע��ʵ��Ļ�������������ע�����

��ϰ��ϵ�д�
 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
�����Ŀ